Clinical Studies – Targeting Acute and Chronic Thromboembolic Disorders
COSIMO: Patients with active cancer changing to Xarelto for prevention of recurrent VTE
Background
- LMWH is the current standard of care as an anticoagulation therapy in people with cancer and are recommended by current guidelines
- However, the burden of care associated with traditional anticoagulation therapies for CAT may explain the high levels of non-adherence to the current guidance in clinical practice
Objective1
- To provide real-world information on treatment satisfaction in patients with active cancer who switch from LMWH or VKA to Xarelto for the treatment of acute VTE or to prevent recurrent VTE
Study design1
- COSIMO was a prospective, non-interventional, single-arm cohort study which recruited 505 patients in Europe, Canada and Australia. Adults with active cancer who are switching to Xarelto having received LMWH / VKA for the treatment and secondary prevention of recurrent VTE for at least 4 weeks were eligible
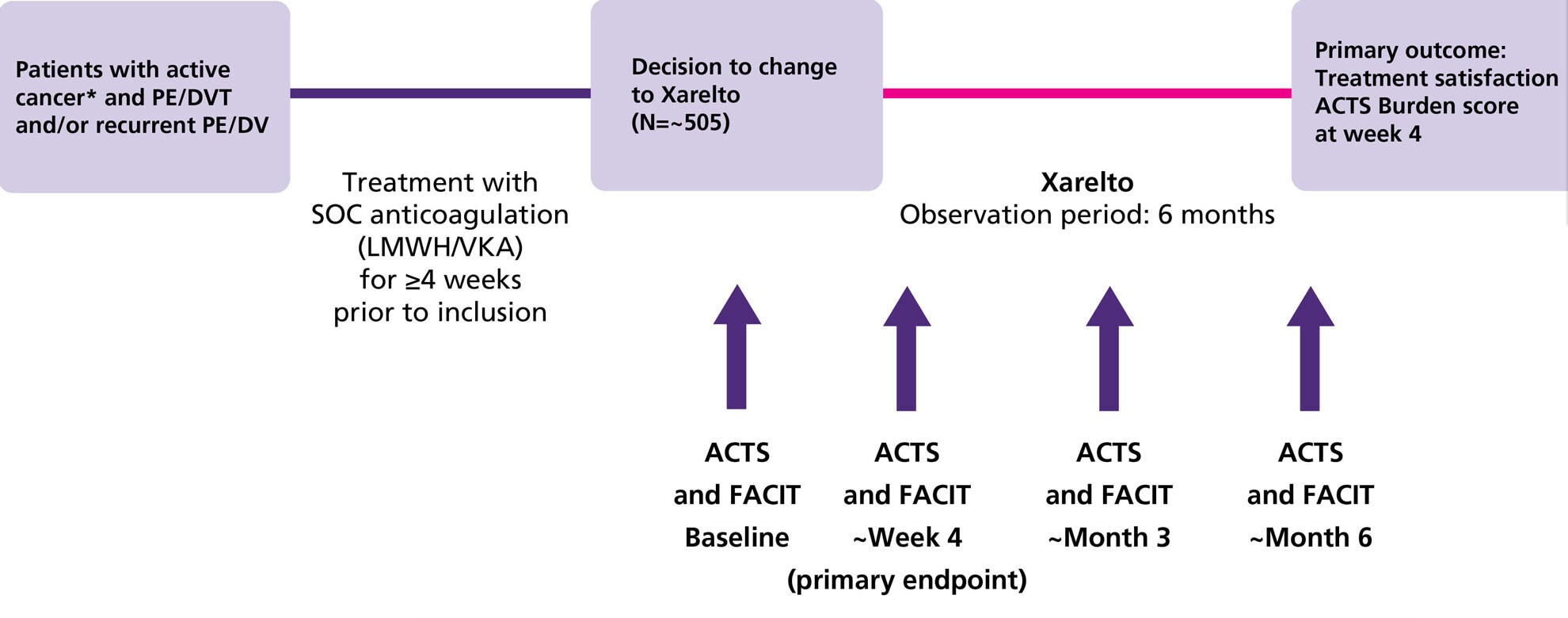
Endpoints1
Primary efficacy outcome
- Treatment satisfaction (ACTS Burdens score)
Safety outcomes
- Incidence of bleeding
- Thromboembolic events
- AEs and serious AEs
Secondary efficacy outcomes
- Patient preferences with regard to convenience attributes
- Change in ACTs Burdens sore at month 3 and month 6
- Change in HRQoL
Key findings2
- Patient-reported ACTS Burdens scores were significantly improved at week 4 compared with baseline in the ACTS week 4 analysis set, (n=381) signifying a decrease in perceived treatment burdens following a change to Xarelto
- The change in ACTS Burdens scores was also significant at month 3 and month 6 compared with baseline
ACTS, anti-clot treatment scale; AE, adverse event; CAT, cancer-associated thrombosis ; HRQoL, health-related quality of life; LMWH , low-molecular weight heparin; SAE, serious adverse event; VTE , venous thromboembolism; VKA , vitamin K antagonist
*Diagnosis or treatment of cancer in the previous <6 months, or recurrent or metastatic cancer other than fully treated basal cell or squamous cell carcinoma of the skin.1
PP-XAR-ALL-1828-1
References
- Cohen AT, et al. Thromb J. 2018;16:21. Cohen AT, et al. Thromb J. 2018;16:21. Return to content
- Cohen AT, et al. Patient-reported outcomes associated with switching to rivaroxaban for the treatment of venous thromboembolism in patients with active cancer. Poster 1774P presented at the European Society of Medical Oncology (ESMO) congress, 27 September – 1 October 2019, Barcelona, Spain. Cohen AT, et al. Patient-reported outcomes associated with switching to rivaroxaban for the treatment of venous thromboembolism in patients with active cancer. Poster 1774P presented at the European Society of Medical Oncology (ESMO) congress, 27 September – 1 October 2019, Barcelona, Spain. Return to content







