Clinical Studies – Targeting Acute and Chronic Thromboembolic Disorders
EINSTEIN programme
Treatment of Venous Thromboembolism:
the EINSTEIN Programme
The EINSTEIN clinical trial programme comprises four randomised Phase 3 studies of rivaroxaban for the treatment of VTE and the long-term prevention of recurrent VTE. It is the only clinical programme that has investigated a new oral anticoagulant for the treatment of acute DVT and treatment for acute PE in separate trials. It also comprises of the first completed trial of an oral anticoagulant in children, the largest study to date in children with VTE.
- EINSTEIN DVT : rivaroxaban versus enoxaparin plus a vitamin K antagonist ( VKA ) in the treatment of acute DVT without symptomatic PE
- EINSTEIN PE: rivaroxaban versus enoxaparin plus a VKA in the treatment of acute PE with or without symptomatic DVT
- EINSTEIN EXT: rivaroxaban versus placebo in the long-term prevention of recurrent, symptomatic VTE in patients who had already received 6–12 months of anticoagulant treatment for DVT or PE
- EINSTEIN JUNIOR: rivaroxaban vs continuation of heparin/VKA in the prevention of recurrent VTE in children aged 0–17 years
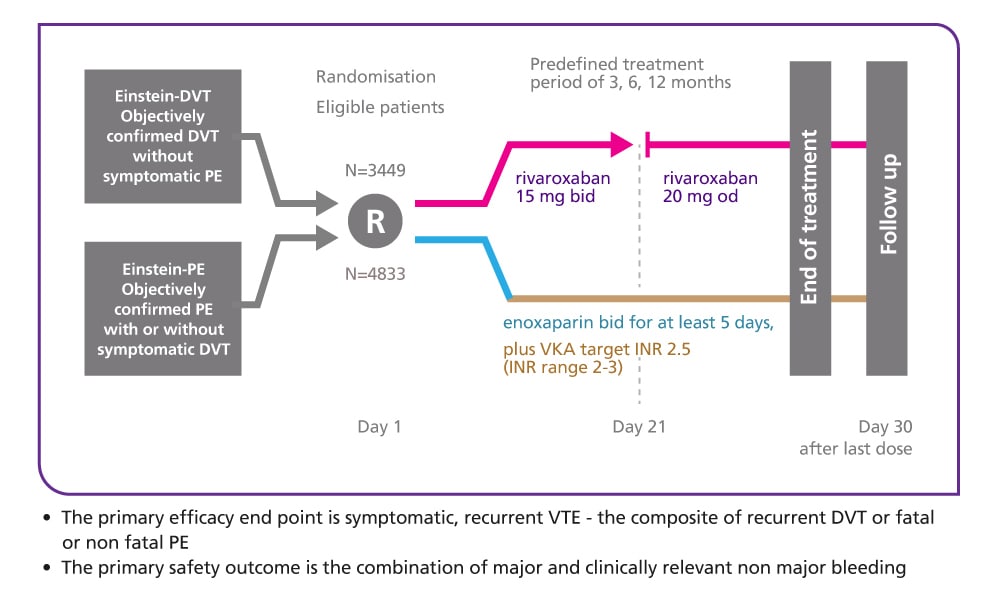
EINSTEIN DVT design5
Single-drug therapy with oral rivaroxaban versus dual-drug therapy with subcutaneous enoxaparin and oral VKA
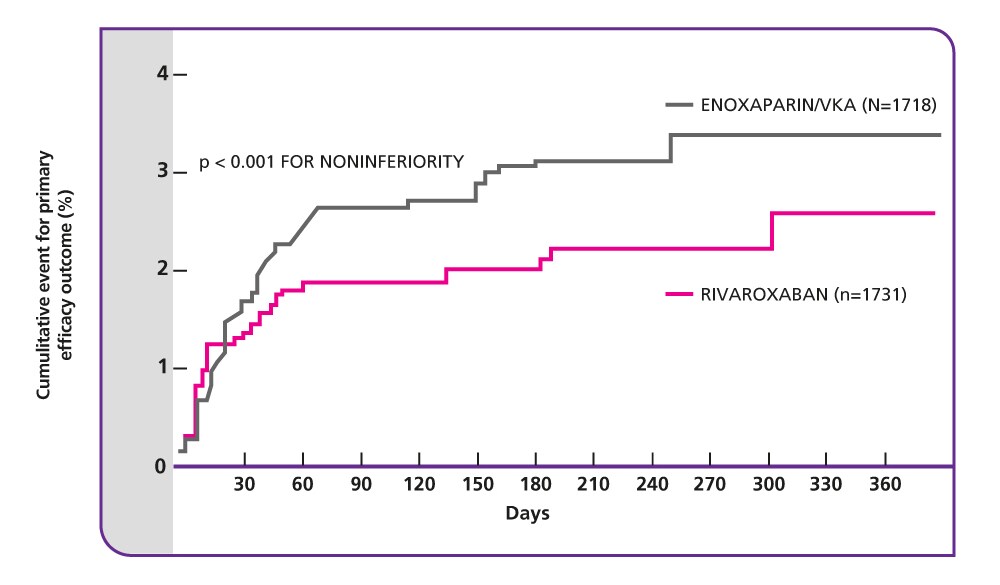
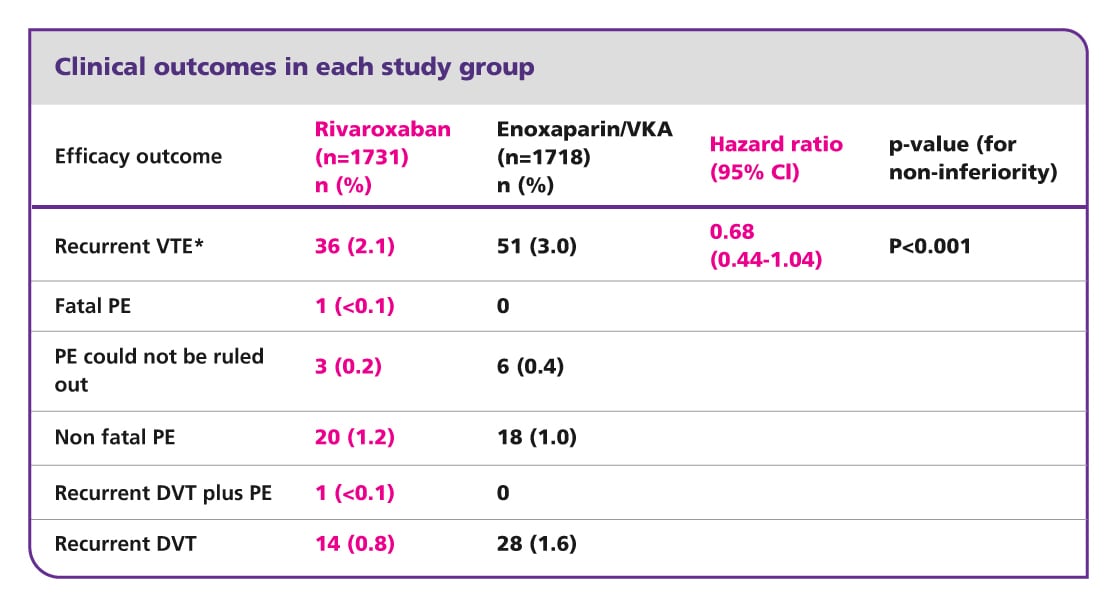
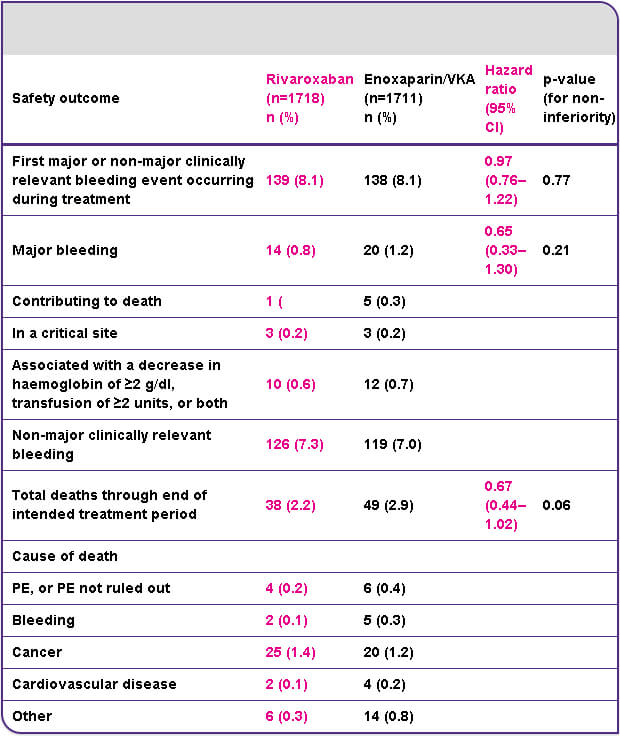
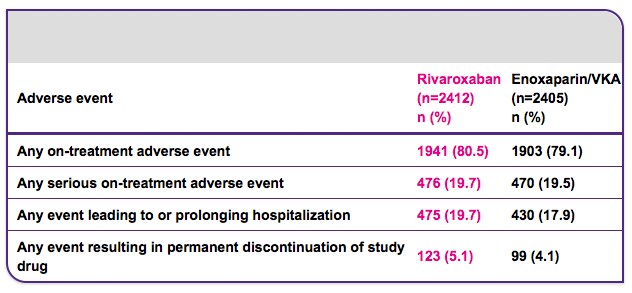
EINSTEIN DVT results5
Efficacy and safety of rivaroxaban in the treatment of deep vein thrombosis
EINSTEIN PE design6
Single-drug therapy with oral rivaroxaban versus dual-drug therapy with subcutaneous enoxaparin and oral VKA
EINSTEIN PE results6
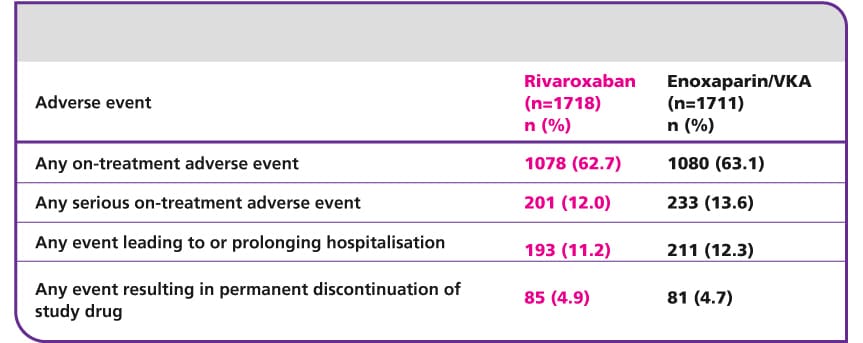
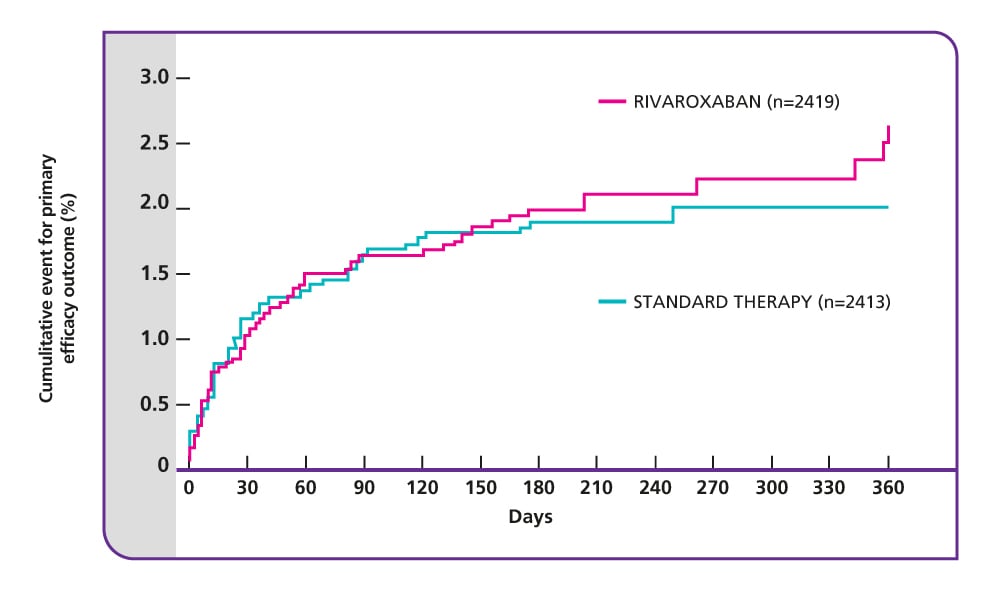
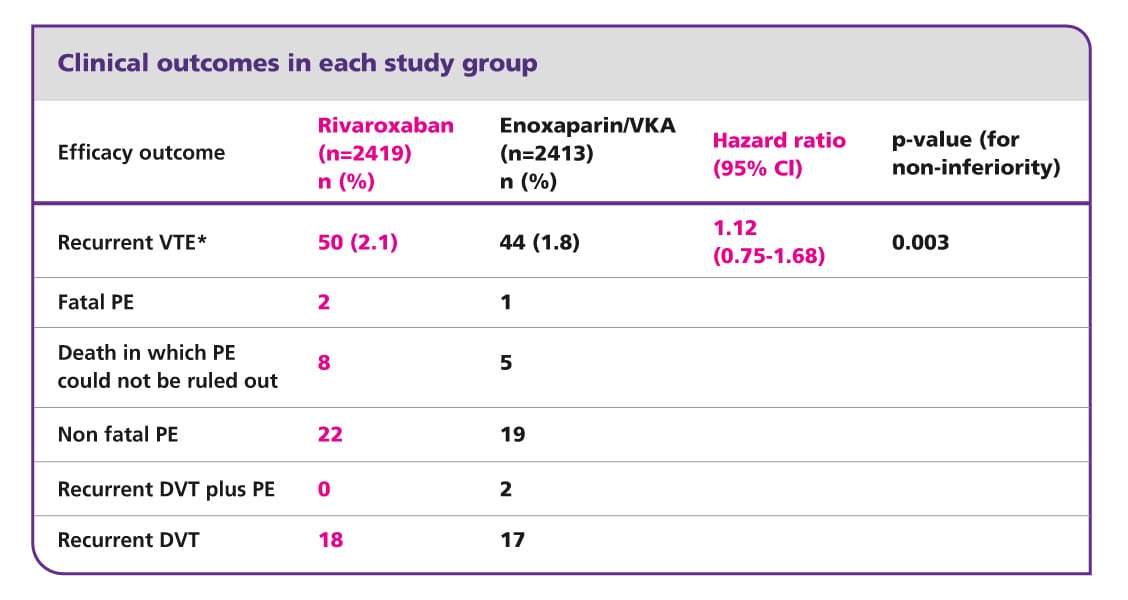
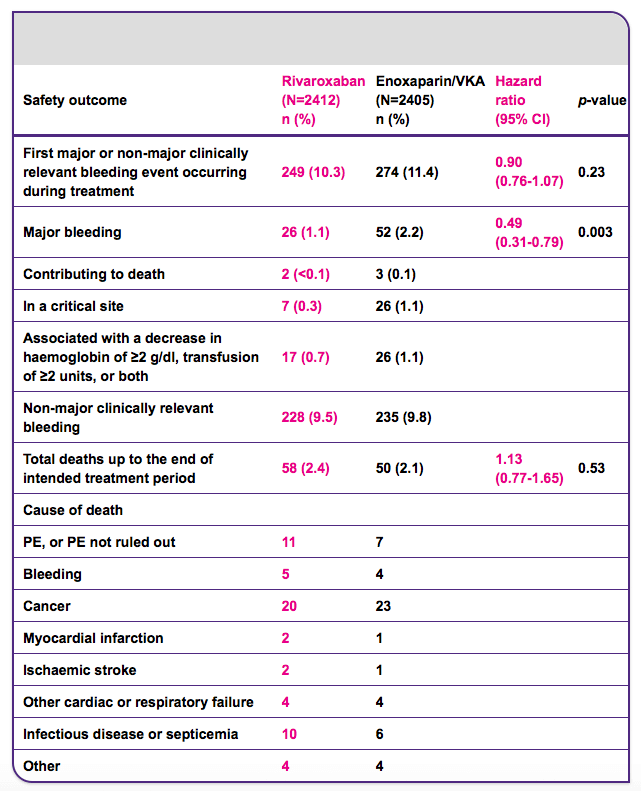
Efficacy and safety of rivaroxaban in the treatment of pulmonary embolism
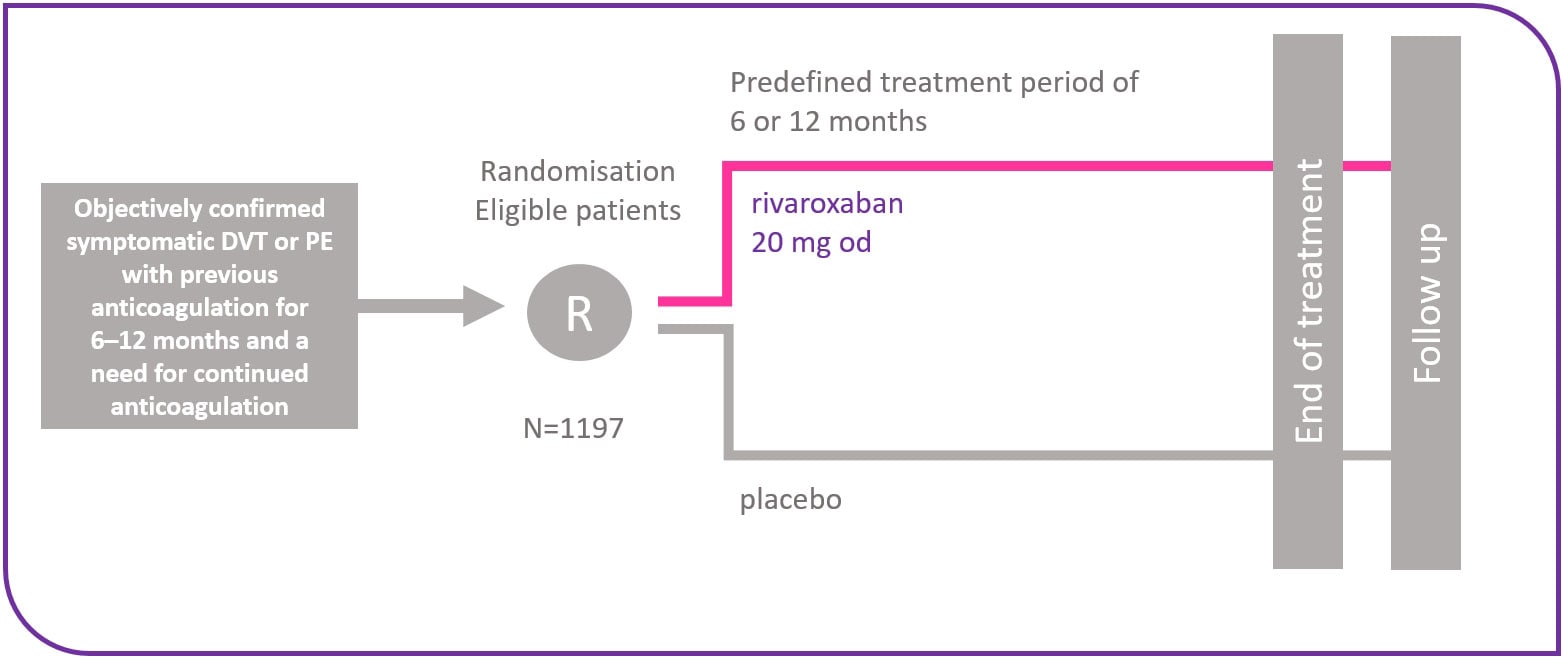
EINSTEIN EXT design5
The long-term prevention of recurrent, symptomatic VTE
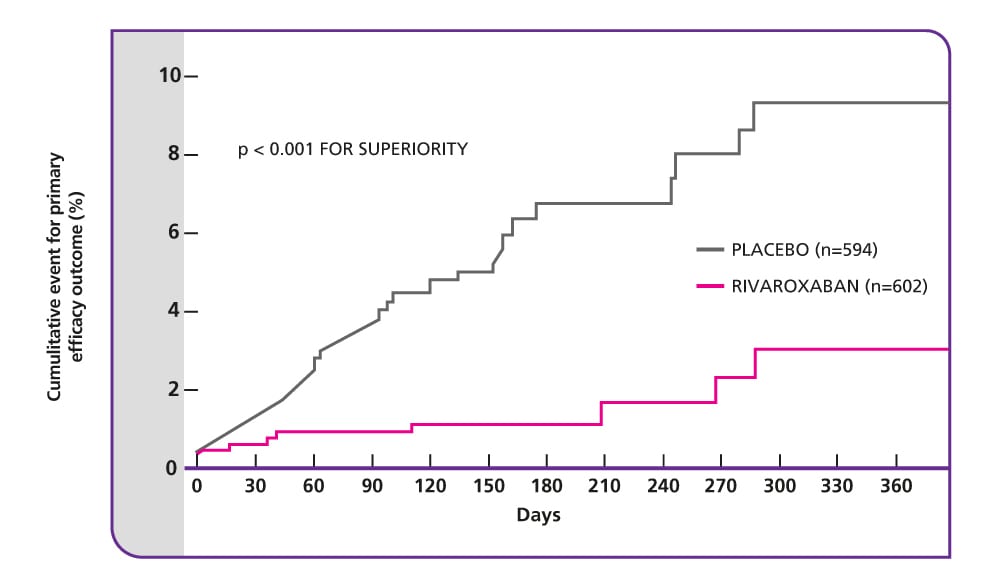
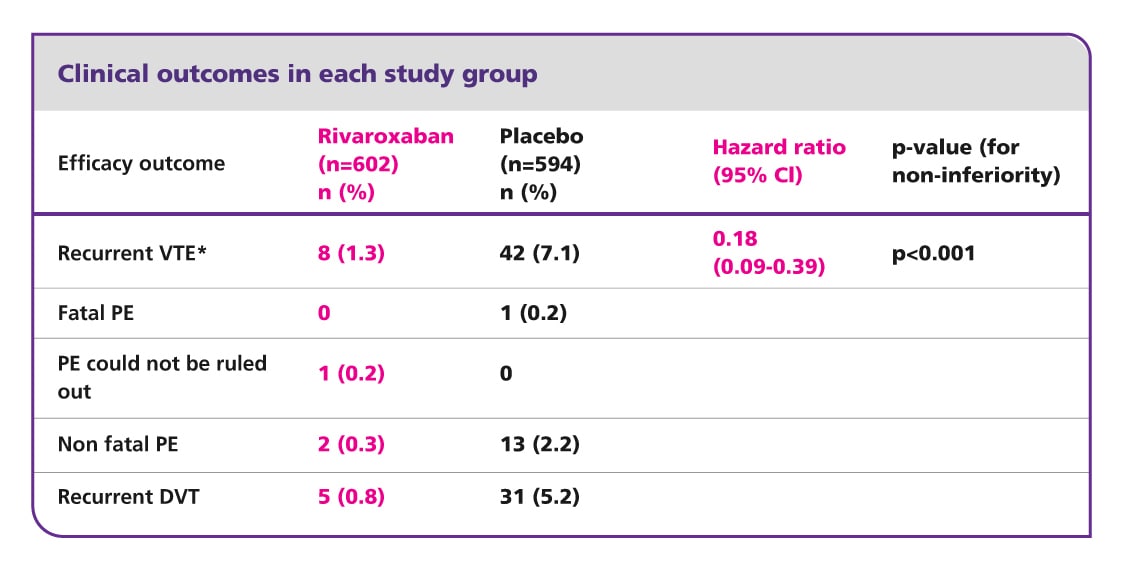
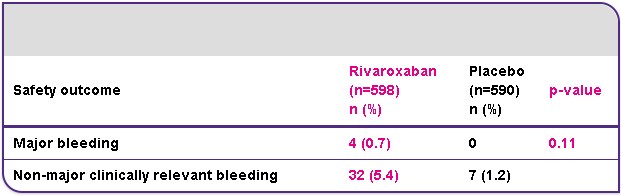
EINSTEIN EXT results5
Efficacy and safety of rivaroxaban for prevention of recurrent venous thromboembolism
EINSTEIN Junior design7
Bodyweight adjusted rivaroxaban in a 20 mg-equivalent dose versus the standard of care* in children
* Following 5–9 days of LMWH/unfractionated heparin/fondaparinux, patients either continued treatment with or were switched to a VKA.7
EINSTEIN Junior results7
Efficacy and safety of rivaroxaban for the prevention of recurrent venous thromboembolism
PP-XAR-ALL-1822-1
References
- Steiff MB et al. J Thromb Thrombolysis. 2016;41:32-67. Return to content
- Ageno W, et al. Chest. 2012;141:e44S–e88S. Return to content
- Cohen AT, et al. Thromb Haemost. 2007;98:756–764. Return to content
- Kearon C. Circulation. 2003;107(23 suppl 1):I22–I30. Return to content
- The EINSTEIN Investigators. N Engl J Med. 2010;363:2499-510. Return to content
- The EINSTEIN-PE Investigators. N Engl J Med. 2012;366:1287-1297. Return to content
- Male C et al. Lancet Haematol 2020;7:e18–e27. Return to content