Clinical Studies – Targeting Acute and Chronic Thromboembolic Disorders
RECORD Programme: 4 Phase III Studies on Total Knee and Total Hip Replacement Surgery
The RECORD Programme:
RECORD1, 2, 3 and 4 Trials
The RECORD (REgulation of Coagulation in ORthopaedic Surgery to Prevent Deep Vein Thrombosis and Pulmonary Embolism) programme comprised four randomised clinical trials of rivaroxaban for the prevention of venous thromboembolism in adult patients after total hip replacement (THR) or total knee replacement (TKR)1-4
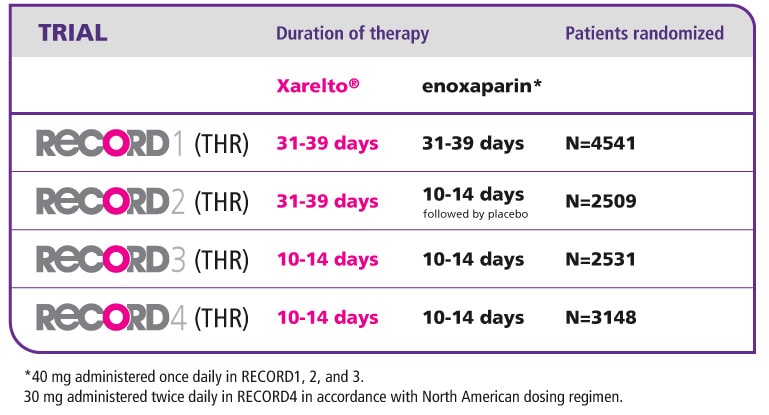
- In the RECORD1 study, extended regimens of oral, once-daily rivaroxaban 10 mg and a once-daily injection of enoxaparin in a 40 mg dose were compared for the prevention of VTE in patients undergoing elective THR. Rivaroxaban was initiated 6–8 hours after wound closure and continued for 31–39 days. Enoxaparin was initiated 12 hours before surgery, restarted 6–8 hours after surgery and then continued for 31–39 days.
- In the RECORD2 study, to investigate the benefits of an extended treatment regimen in the prevention of VTE in patients undergoing elective THR, oral, once-daily rivaroxaban 10 mg was initiated 6–8 hours after wound closure and continued for 31–39 days. This was compared with a short-term regimen of a once-daily injection of enoxaparin in a 40 mg dose. Enoxaparin was given 12 hours before surgery, restarted 6–8 hours after surgery, continued for 10–14 days, and followed by placebo until day 31-39.
- In the RECORD3 study, oral, once-daily rivaroxaban 10 mg was compared with a once-daily injection of enoxaparin in a 40 mg dose for the prevention of VTE in patients undergoing elective TKR. Rivaroxaban was initiated 6–8 hours after wound closure. Enoxaparin was initiated 12 hours before surgery and given again 6–8 hours after wound closure. Both study medications were continued at least to day 10 and up to day 14.
- In the RECORD4 study, a short-term treatment regimen with oral, once-daily rivaroxaban 10 mg was compared with a twice-daily injection of enoxaparin 30 mg (approved US dosing) in the prevention of VTE in patients undergoing elective TKR. Rivaroxaban was initiated 6–8 hours after wound closure and enoxaparin was initiated 12–24 hours after wound closure. Both study medications were continued at least to day 10 and up to day 14.
Background
- Venous thromboembolism ( VTE ), which encompasses both deep vein thrombosis ( DVT ) and pulmonary embolism ( PE ), is one of the most frequently occurring serious complications after elective hip or knee replacement surgery
- Approximately 40–85% of orthopaedic surgery patients are thought to be at risk of VTE if appropriate thromboprophylaxis is not administered
- Guidelines recommend VTE prophylaxis after major orthopaedic surgery with non-vitamin K antagonist oral anticoagulants (NOACs). They also recommend using low molecular weight heparins ( LMWH ), fondaparinux or vitamin K antagonists ( VKA ), but these traditional anticoagulants have limitations:
- Parenteral administration: LMWH, fondaparinux
- Routine coagulation monitoring: LMWH, VKAs
- Multiple food and drug interactions: VKAs
- With a trend towards shorter hospital stays and thromboprophylaxis recommended for up to 35 days after THR and at least 10 days after TKR, a simple, effective, oral anticoagulant would be beneficial, especially in the outpatient setting
Objective of the RECORD programme
The RECORD programme compared the efficacy and safety of oral rivaroxaban with subcutaneous enoxaparin for the prevention of VTE after THR or TKR surgery in adult patients.1-4
Study design
The RECORD programme comprised four randomised, double-blinded, active comparator-controlled, multinational Phase 3 clinical trials in patients scheduled to undergo elective THR or TKR surgery. More than 12,500 patients from 39 countries were randomly assigned to one of regimens detailed below in Table 1.
Endpoints
The endpoints were the same for all four RECORD studies:
- The primary efficacy endpoint was total VTE defined as the composite of DVT , non-fatal PE and death from any cause
- The primary safety outcome was the incidence of major bleeding
PP-XAR-ALL-1823-1
References
- Eriksson BI, et al. N Engl J Med. 2008;358:2765–2775. Eriksson BI, et al. N Engl J Med. 2008;358:2765–2775. Return to content
- Kakkar AK, et al. Lancet. 2008;372:31–39. Kakkar AK, et al. Lancet. 2008;372:31–39. Return to content
- Lassen MR, et al. N Engl J Med. 2008;358:2776–2786. Lassen MR, et al. N Engl J Med. 2008;358:2776–2786. Return to content
- Turpie AGG, et al. Lancet. 2009;373:1673–1680. Turpie AGG, et al. Lancet. 2009;373:1673–1680. Return to content







