Clinical Studies – Targeting Acute and Chronic Thromboembolic Disorders
SELECT-D: A randomised control trial of Xarelto vs dalteparin in patients with active cancer
Background1
- The risk of recurrent VTE is increased at least two-fold in patients with cancer compared to those without
- For more than a decade, subcutaneous low-molecular weight heparin ( LMWH ) has been the standard of care
Objective1
- To assess if an oral factor Xa inhibitor, Xarelto, would offer an alternative treatment for VTE in patients with cancer
Study design1
- A multicentre, randomised, open-label, pilot trial in the United Kingdom to investigate patients with active cancer who had symptomatic PE , incidental PE or DVT . Patients were treated with either dalteparin or Xarelto
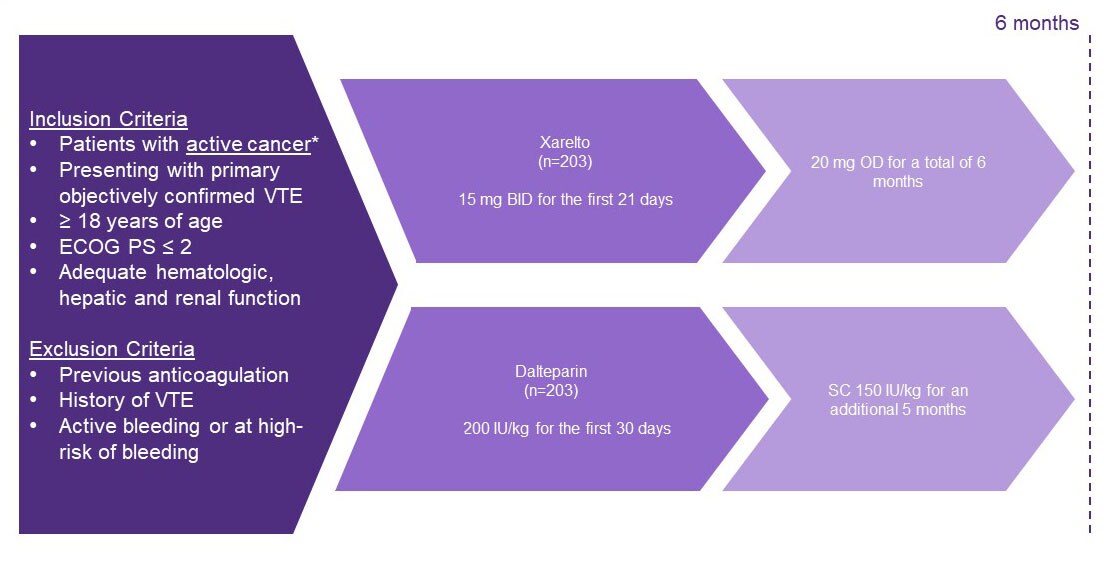
Endpoints1
Primary efficacy outcome
- VTE recurrence
Primary safety outcomes
- Major bleeding
- Clinically non-relevant bleeding
Key findings1
- Over the course of 6 months fewer patients treated with Xarelto experienced a recurrent VTE compared to dalteparin (4% vs 11.0% respectively).
- Only 54% of patients completed the 6-month duration of study with a median duration of treatment of 5.8 and 5.9 months for dalteparin and Xarelto respectively
- 4% of patients receiving dalteparin had major bleeds compared with 6% of patients treated with Xarelto.
- Incidence of fatal bleeding was the same in both groups (0.5%)
- 7 patients receiving dalteparin experienced CRNMB compared with 25 patients receiving Xarelto (3-fold relative increase).
- Overall survival at 6 months was 75% for Xarelto (95% CI 69%–81%) and 70% for dalteparin (95% CI 63%–76%).
BID, twice daily; ECOG PS, Eastern Cooperative Oncology Group performance status; LMWH , low-molecular weight heparin; OD, once daily; SC, subcutaneous ; VTE , venous thromboembolism;
*Active cancer was defined as a diagnosis of cancer (other than basal-cell or squamous-cell skin carcinoma) within the previous 6 months, recurrent or metastatic cancer, or cancer not in complete remission.
PP-XAR-ALL-1826-1
References
- Young AM, et al. J Clin Oncol. 2018;36:2017–2023. Young AM, et al. J Clin Oncol. 2018;36:2017–2023. Return to content







