Clinical Studies – Targeting Acute and Chronic Thromboembolic Disorders
SELECT-D extension: Treatment of cancer-associated VTE 12 month outcomes of placebo vs Xarelto® randomisation of the SELECT-D trial.
Background
- The SELECT-D trial demonstrated that Xarelto had a greater effect on reduction in VTE vs dalteparin, but increased bleeding compared with dalteparin in cancer patients at 6 months
- Uncertainty around optimal duration of anticoagulation use remains in patients with active cancer
Objective1
- To assess VTE recurrence and bleeding, with anticoagulation or not, beyond 6 months in a long-term extension of the SELECT-D trial
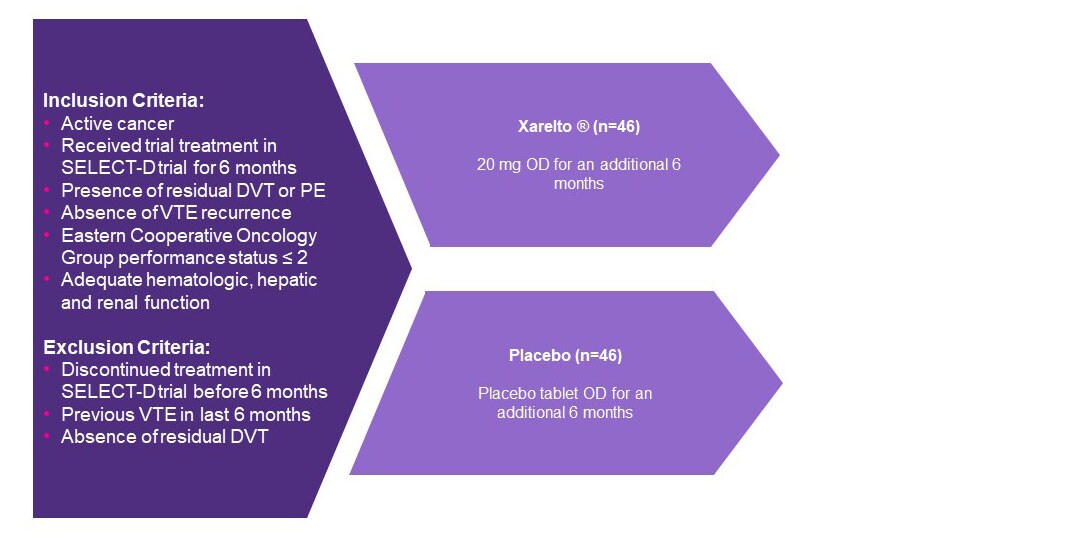
Study design1
- At around 6 months post-randomisation in the SELECT-D trial, patients with CAT with residual DVT or had presented with a PE were randomly assigned to Xarelto 20 mg OD or placebo.
Endpoints1
Primary efficacy outcome
- VTE recurrence at 12 months after first randomisation
Secondary efficacy outcomes
- Major bleeding
- CRNMB
- Overall survival
- VTE recurrence at 12 months for the patient subgroup with no RVDT
Key findings1
- After 6 months only 4% of patients treated with Xarelto had a VTE recurrence (HR 0.32; 95% CI, 0.06-1.58) vs placebo (14%). There was a numerically lower cumulative VTE recurrence rate for Xarelto versus placebo
- The trial was underpowered to detect a statistically significant reduction in recurrent VTE with extended anticoagulation beyond 6 months
- Major bleeding rates were 0% with placebo and 5% with Xarelto (95% CI, 1–18)
- CRNMB were 0% with placebo and 4% with Xarelto (95% CI, 1–17)
- After 6 months, OS for Xarelto-treated patients was 89% (95% CI, 75–95) and 87% (95% CI, 73–94) for placebo patients (HR=1.16; 95% CI, 0.36–3.81)
- Absence of RVDT defined a low VTE recurrence risk group (log rank P=.03)
CAT, cancer-associated thrombosis ; CI, confidence interval; CRNMB, clinically relevant non-major bleeding; DVT , deep vein thrombosis; HR, hazard ratio; OD, once daily; OS, overall survival; PE , pulmonary embolism; RVDT, residual deep vein thrombosis; VTE , venous thromboembolism.
PP-XAR-ALL-1827-1
References
- Marshall A, et al. J Thromb Haemost. 2020. doi: 10.1111/jth.14752. Marshall A, et al. J Thromb Haemost. 2020. doi: 10.1111/jth.14752. Return to content







