Clinical Studies – Targeting Acute and Chronic Thromboembolic Disorders
VOYAGER PAD: A randomised control trial of Xarelto® 2.5 mg BID plus aspirin vs aspirin in patients with symptomatic PAD post-revascularisation
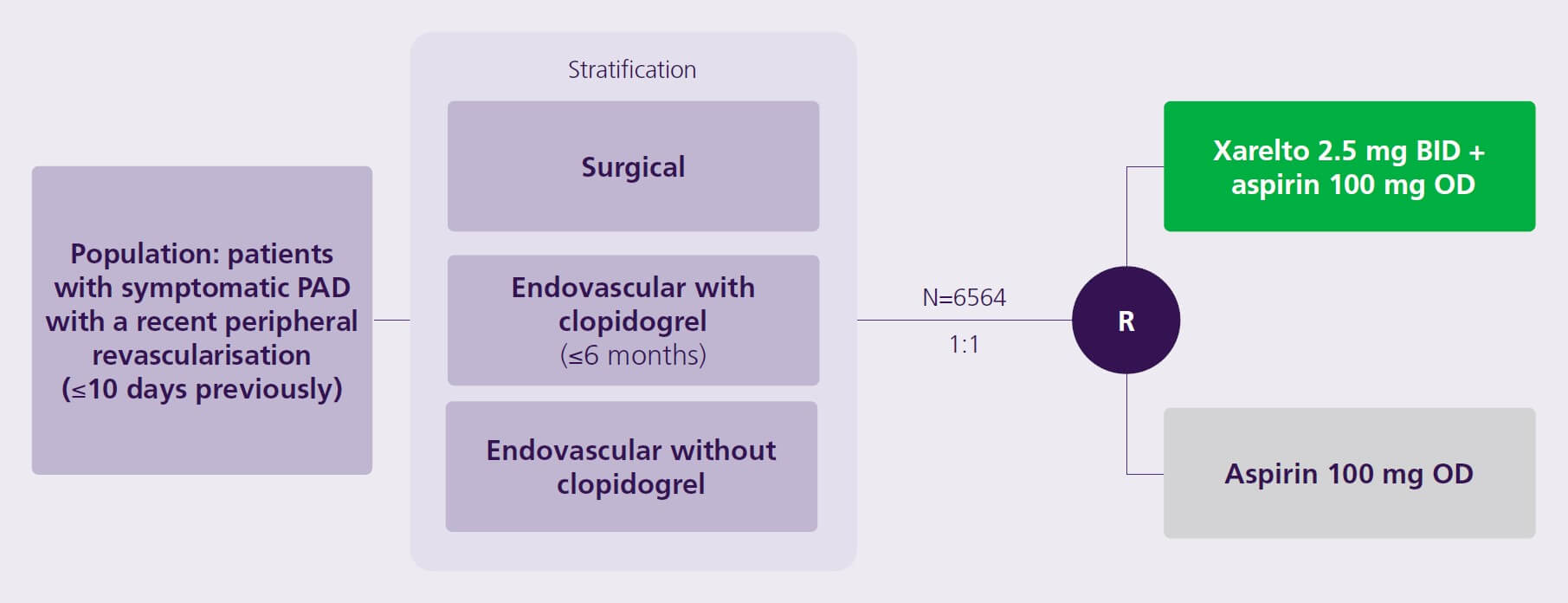
A Phase 3, multicentre, double-blind, placebo-controlled, randomised clinical trial to assess the efficacy and safety of Xarelto 2.5 mg BID plus aspirin vs aspirin in reducing major adverse vascular event risk in patients with symptomatic PAD following recent revascularisation1
Background1
- Patients with PAD who have undergone lower-extremity revascularisation are at high risk for limb ischaemia and major cardiovascular events
- VOYAGER PAD is only large, randomised study to demonstrate a clinically relevant benefit of antithrombotic treatment for patients with symptomatic PAD undergoing lower-extremity revascularisation
Objective1
- To investigate whether Xarelto 2.5 mg BID plus aspirin is effective and safe in patients with symptomatic PAD post-revascularisation vs aspirin
Study design1
- A Phase 3, multicentre, double-blind, placebo-controlled, randomised clinical trial
Endpoints1
Primary composite efficacy outcome
- Acute limb ischaemia, major amputation of vascular aetiology, myocardial infarction, ischaemic stroke or cardiovascular death
Primary safety outcome
- TIMI major bleeding
Secondary efficacy outcomes
- Acute limb ischaemia, major amputation of vascular aetiology, myocardial infarction, ischaemic stroke, or coronary heart disease death
- Unplanned index limb revascularisations for recurrent limb ischaemia
- Vascular hospitalisations for a peripheral or coronary event of a thrombotic nature
- Acute limb ischemia, major amputation of vascular aetiology, myocardial infarction, ischemic stroke or all-cause mortality
- Acute limb ischemia, major amputation of vascular aetiology, myocardial infarction, all stroke or cardiovascular death
- All-cause mortality
- Venous thromboembolism
Secondary safety outcome
- ISTH major bleeding
Key findings1
Unmet need:
- 1 in 5 patients in the aspirin group had the primary composite efficacy outcome at 3 years
In VOYAGER PAD:
- The cumulative incidence of the primary composite efficacy outcome was reduced by 15% with Xarelto 2.5 mg BID plus aspirin vs aspirin (HR: 0.85, 95% CI: 0.76–0.96, p=0.009)
- The risk of unplanned index limb revascularisations for recurrent limb ischaemia was reduced by 12% with Xarelto 2.5 mg BID plus aspirin vs aspirin (HR: 0.88, 95% CI: 0.79–0.99, p=0.03)
- The risk of acute limb ischemia was reduced by 33% with Xarelto 2.5 mg BID plus aspirin vs aspirin (HR: 0.67, 95% CI: 0.55–0.82)
- Bleeding was generally manageable. The primary safety endpoint of TIMI major bleeding was not significantly increased with Xarelto 2.5 mg BID plus aspirin vs aspirin
- No increase in intracranial haemorrhage or fatal bleeding with Xarelto 2.5 mg BID plus aspirin vs aspirin
BID, twice daily; ISTH, International Society on Thrombosis and Haemostasis; OD, once daily; PAD, peripheral artery disease; R, randomisation; TIMI , Thrombolysis in Myocardial Infarction.
PP-XAR-ALL-2119-1
References
- Bonaca MP et al. N Engl J Med 2020;382:1994–2004. Return to content







