The Treatment Choice Today, Confidently Protecting Your Patients Tomorrow1–8
Treatment of PE/DVT and prevention of recurrent VTE
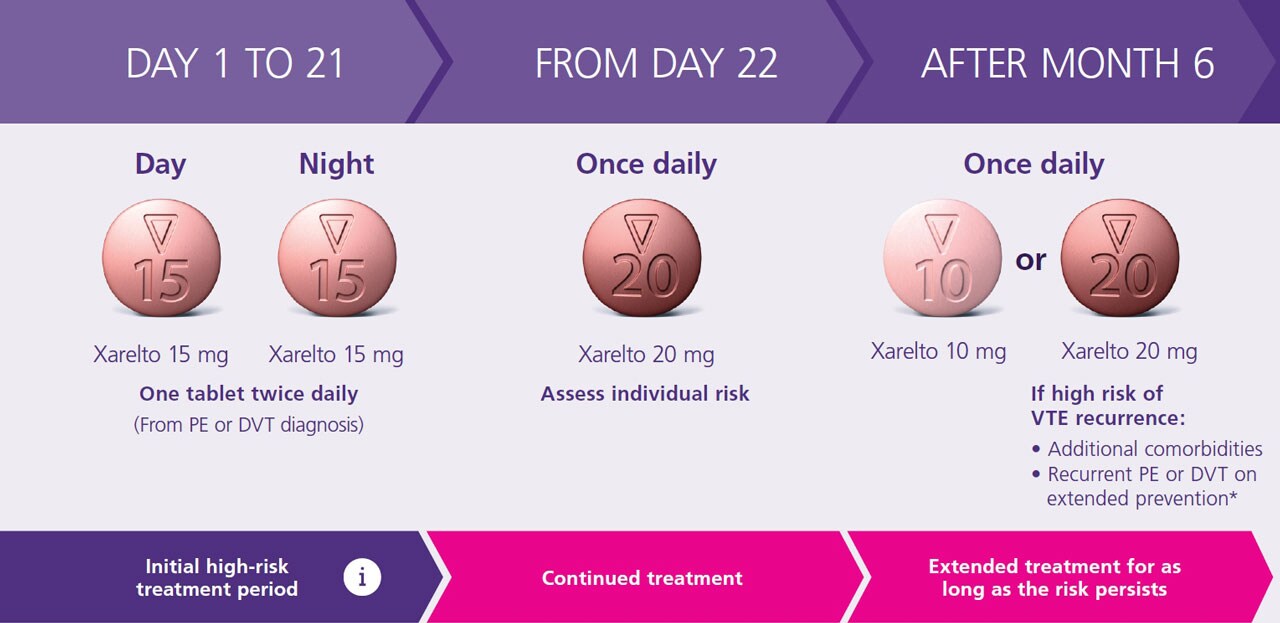
Xarelto® is indicated for the treatment of pulmonary embolism (PE) ,deep vein thrombosis (DVT) and prevention of recurrence in adults1
Xarelto is indicated for the treatment of venous thromboembolism (VTE) and prevention of VTE recurrence children aged less than 18 years after at least 5 days of initial parenteral anticoagulation1
Protection starts with getting to know your patient
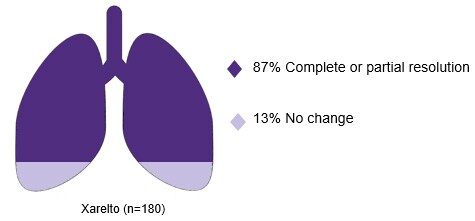
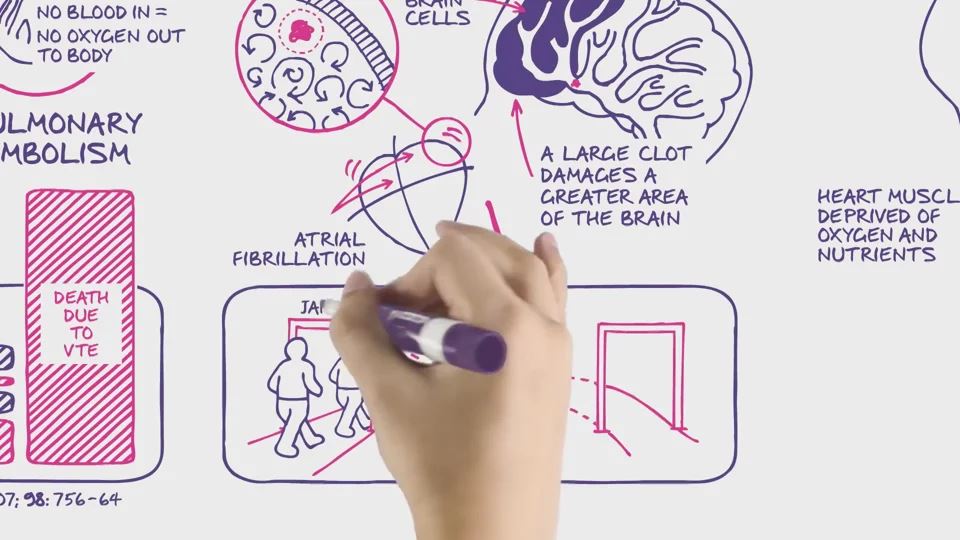
Protect what matters most with fast and effective PE clot resolution
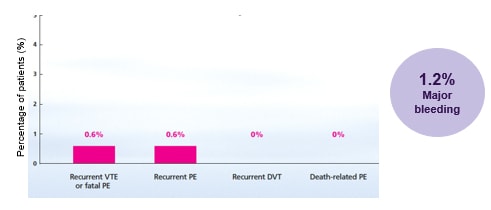
Proven protection from the comfort of their own home
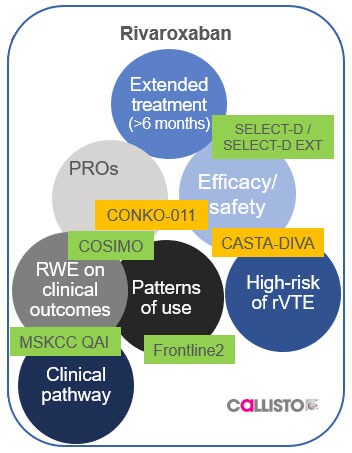
Look after the life ahead for your patients with VTE
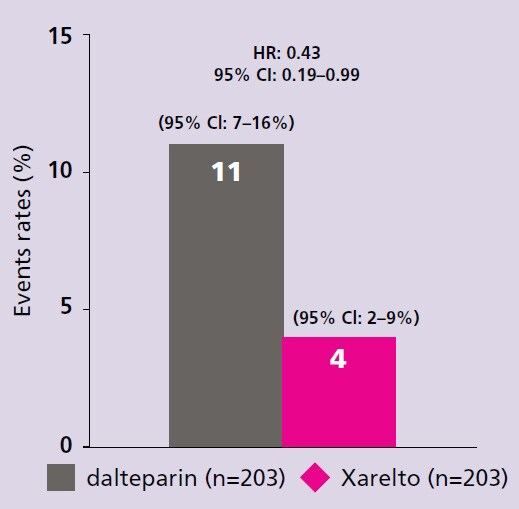
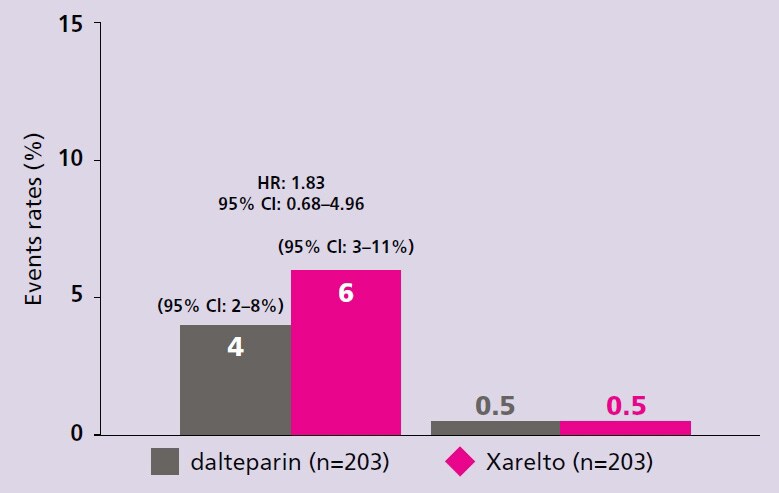
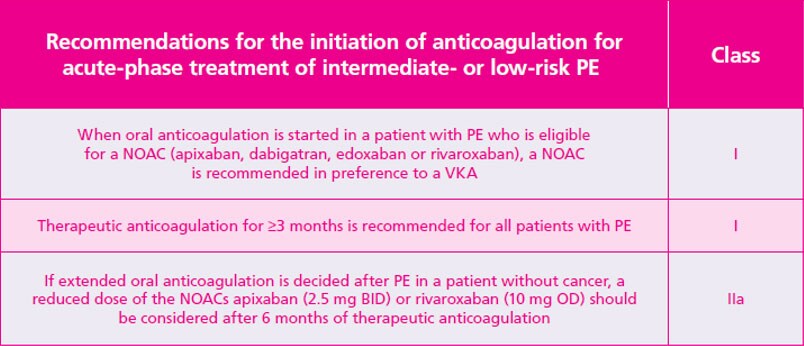
Xarelto is recommended by the ESC for your patients with VTE, including those with active cancer
People like Rose need a dosing regimen that’s right for them
ACTS, anti-clot treatment scale; ASCO, American Society of Clinical Oncology; ARR, absolute risk reduction; BID, twice daily; CAT, cancer-associated thrombosis ; CI, confidence interval; CT, computed tomography; DVT , deep vein thrombosis; ESC, European Society of Cardiology; HR, hazard raio; ISTH, International Society of Thrombosis and Haemostasis; ITAC, The International Initiative on Thrombosis and Cancer; LMWH , low moecular weight heparin; OD, once daily; PE , pumonary embolism; RRR , relative risk reduction; VKA , vitamin K antagonist; VTE , venous thromboembolism.
References
- Bayer AG. Xarelto® (rivaroxaban) Summary of Product Characteristics. Available at: https://www.ema.europa.eu/documents/product-information/xarelto-epar-product-information_en.pdf. Return to content
- Van Es, Douma RA, Kamphuisen PW et al. Clot resolution after 3 weeks of anticolagulant treatment for pulmonary embolism: comparison of computed tomography and perfusion scintigraphy. J Thromb Haemost. 2013;11:679–685. Return to content
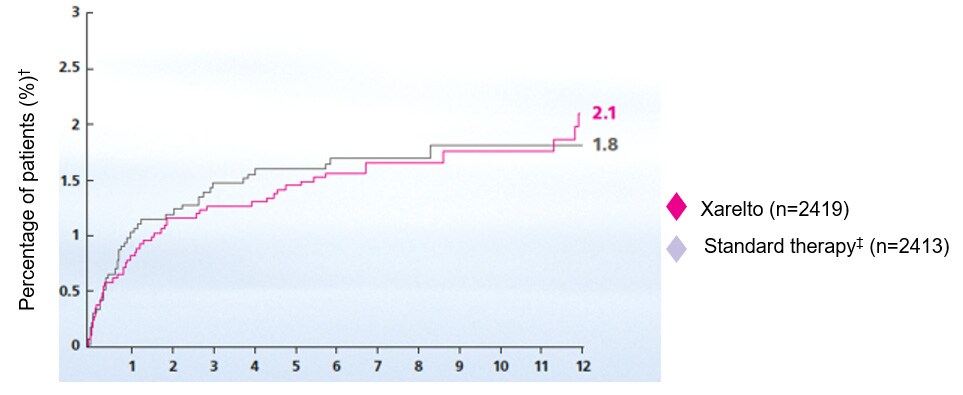
- Buller HR, Prins MH, Lensing AWA et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2019;366:1287–1297. Return to content
- Prins M, Lensing AWA, Bauersachs R et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thrombosis J. 2013;11(1):21–31. Return to content
- Barco S, Schmidtmann I, Ageno W et al. Early discharge and home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban; an international multicentre single-arm clinical trial. Eur Heart J. 2020;41:509–518. Return to content
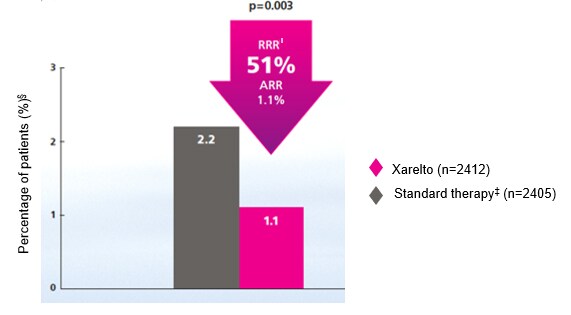
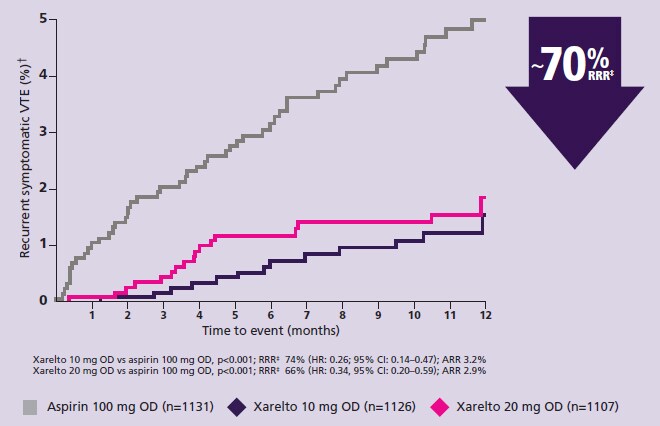
- Weitz JI, Lensing AWA, Prins MH et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211–1222. Return to content
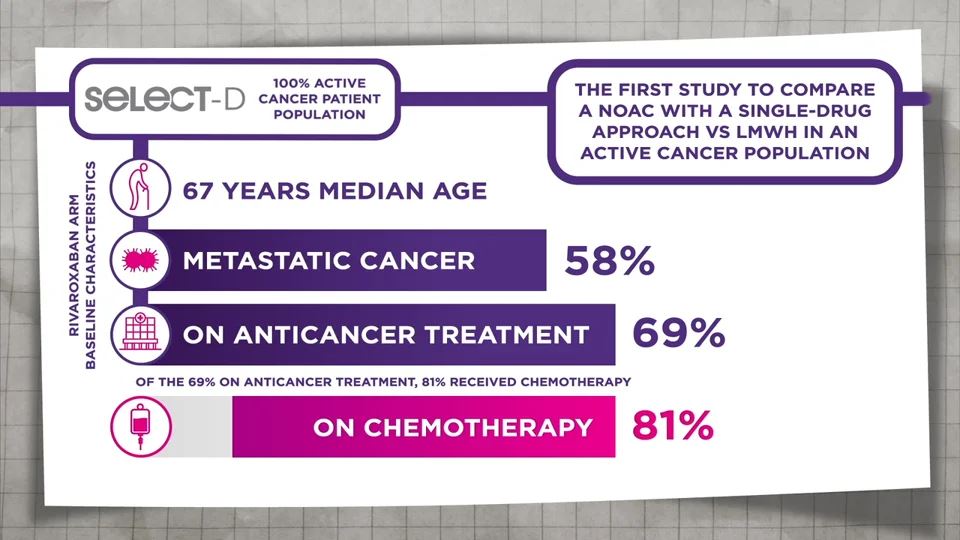
- Young AM, Marshal A, Thirlwall J et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36:2017–2023. Return to content
- Prins MH, Lensing AWA, Brighton TA et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1:e37–e46. Return to content
- Streiff MB, Milentijevic D, McCrae K et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018;93:664–671. Return to content
- Ageno W, Mantovani LG, Haas S et al. Subgroup analysis of patients with cancer in XALIA: A noninterventional study of Rivaroxaban versus standard anticoagulation for VTE. TH Open. 2017;1:e33–e42. Return to content
- Bach M and Bauersachs R. Spotlight on advances in VTE management: CALLISTO and EINSTEIN CHOICE. Thromb Haemost. 2016;116(Suppl 2):S24–S32. Return to content
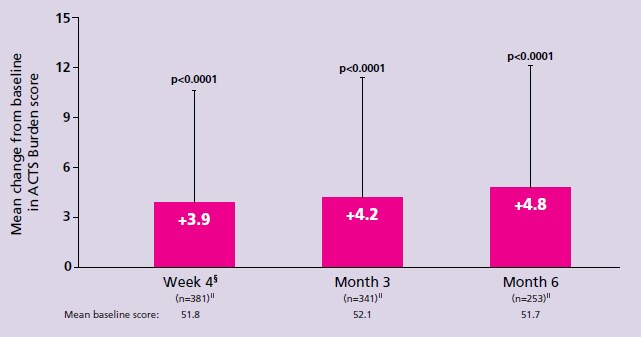
- Cohen AT, et al. Patient-reported outcomes associated with switching to rivaroxaban for the treatment of venous thromboembolism in patients with active cancer. Poster 1774P presented at the European Society of Medical Oncology (ESMO) congress, 27 September – 1 October 2019, Barcelona, Spain. Return to content
- Cohen AT, Maraveyaa A, Beyer-Westendorf J et al. COSIMO – patients with active cancer changing to rivaroxaban for the treatment and prevention of recurrent venous thromboembolism: a non-interventional study. Thromb J. 2018;16:21. Return to content
- Marshall A, Levine M, Hill C et al. Treatment of cancer-associated venous thromboembolism: 12-month outcomes of the placebo versus rivaroxaban randomization of the SELECT-D Trial (SELECT-D: 12m). J Thromb Haemost. 2020;18:905–915. Return to content
- Konstantinides SV, Meyer G, Becattini C et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. Return to content
- Khorana AA, Noble S, Lee AYY et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16:1891–1894. Return to content
- Key NS, Khorana AA, Kuderer NM et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496–520. Return to content
- Farge D, Frere C, Connors JM et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;e566–e581. Return to content
- Limone BL, Hernandez A, Michalak D et al. Timing of recurrent venous thromboembolism early after the index event: A meta-analysis of randomized controlled trials. Thromb Res. 2013;132:420–426. Return to content
RELATED PODCAST
CAD or symptomatic PAD discussion