Xarelto® Tailored Protection for Your Cardio-Vascular Patients1
Prevention of atherothrombotic events in chronic CAD
Xarelto is indicated for the prevention of atherothrombotic events in adults with coronary artery disease (CAD) at high risk of ischaemic events.
It’s time to give your high-risk patients a new standard of care
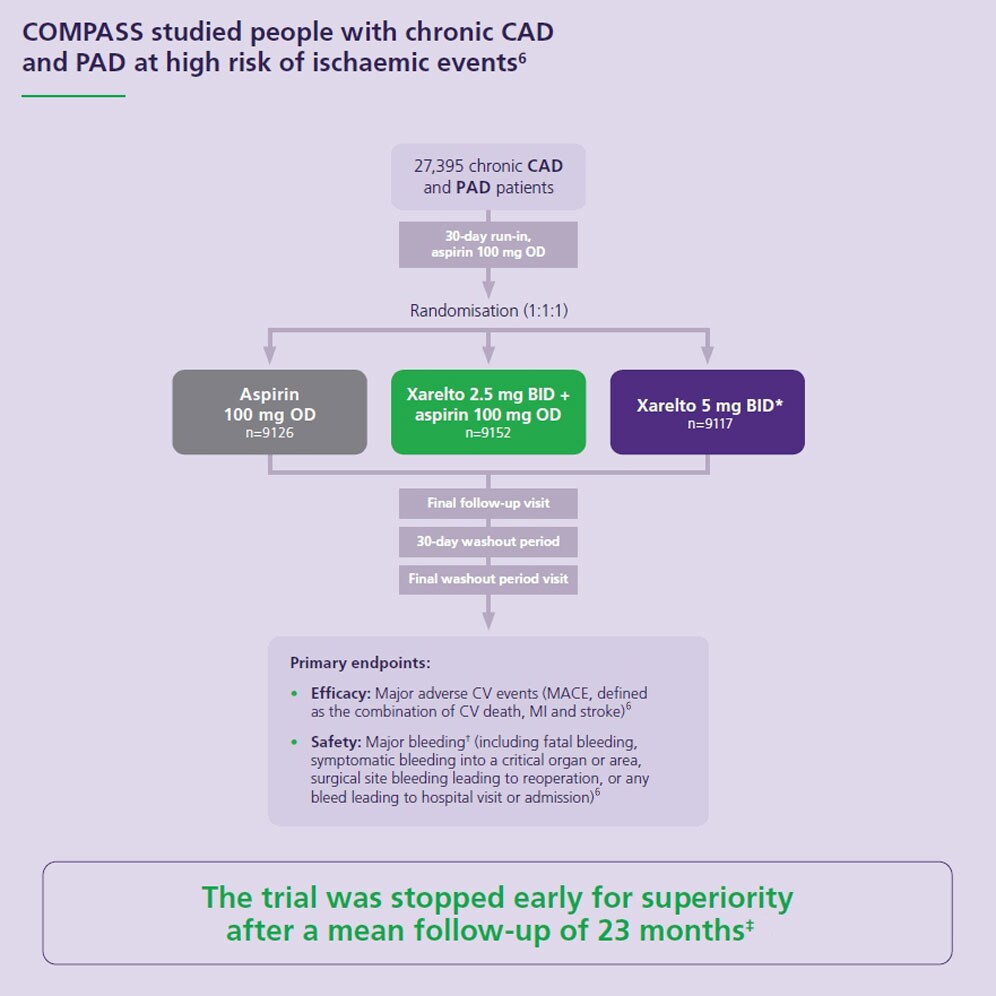
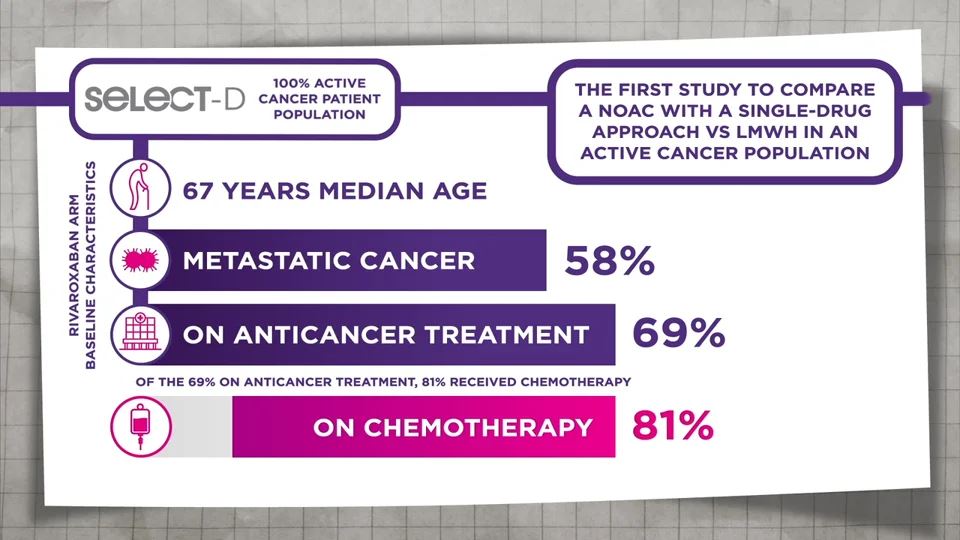
COMPASS – a landmark study for patients with chronic CAD or PAD*
Isn’t life the outcome that matters most for your patients with CAD?
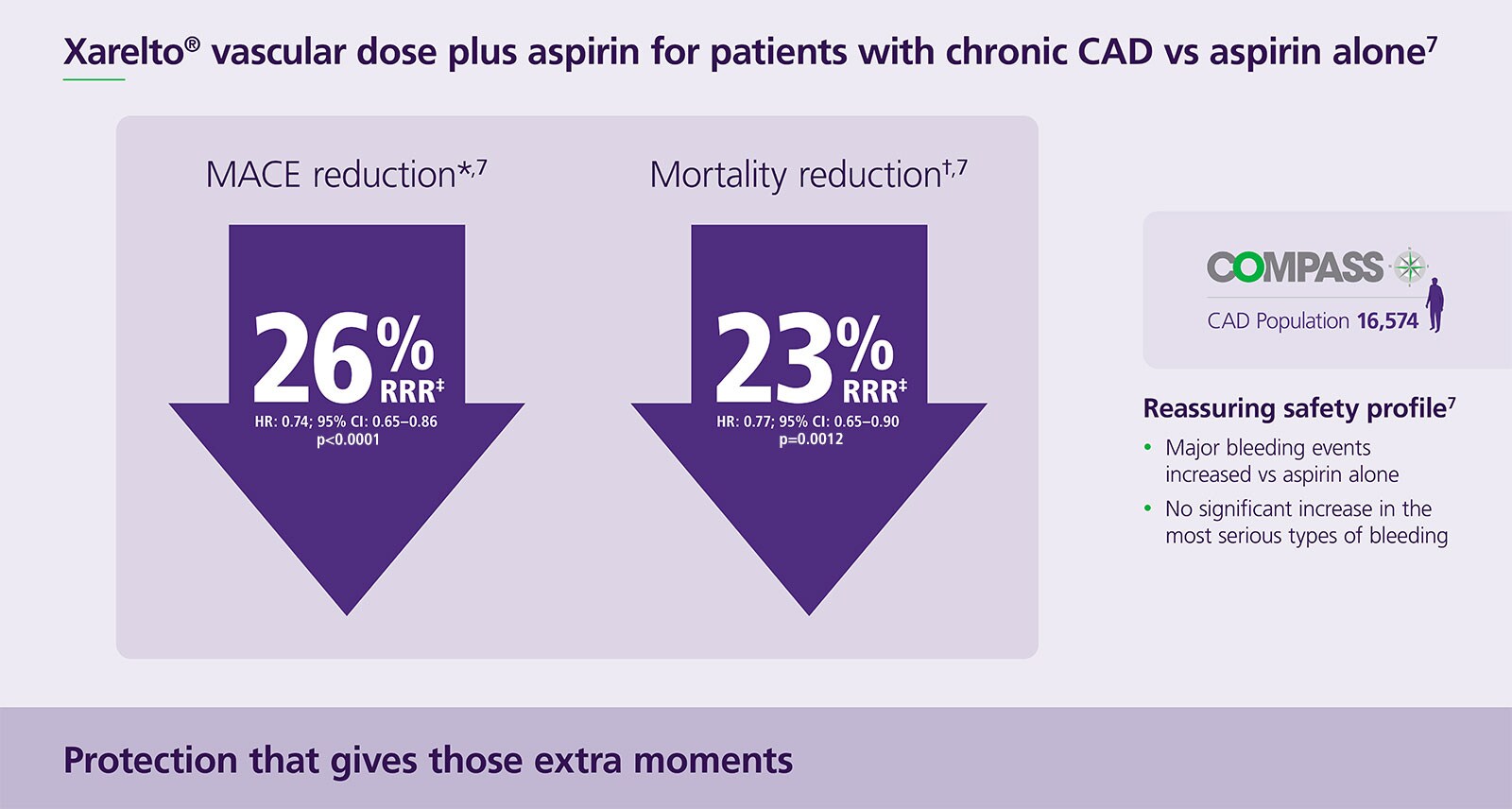
Superior reduction in all-cause mortality and MACE vs aspirin alone7
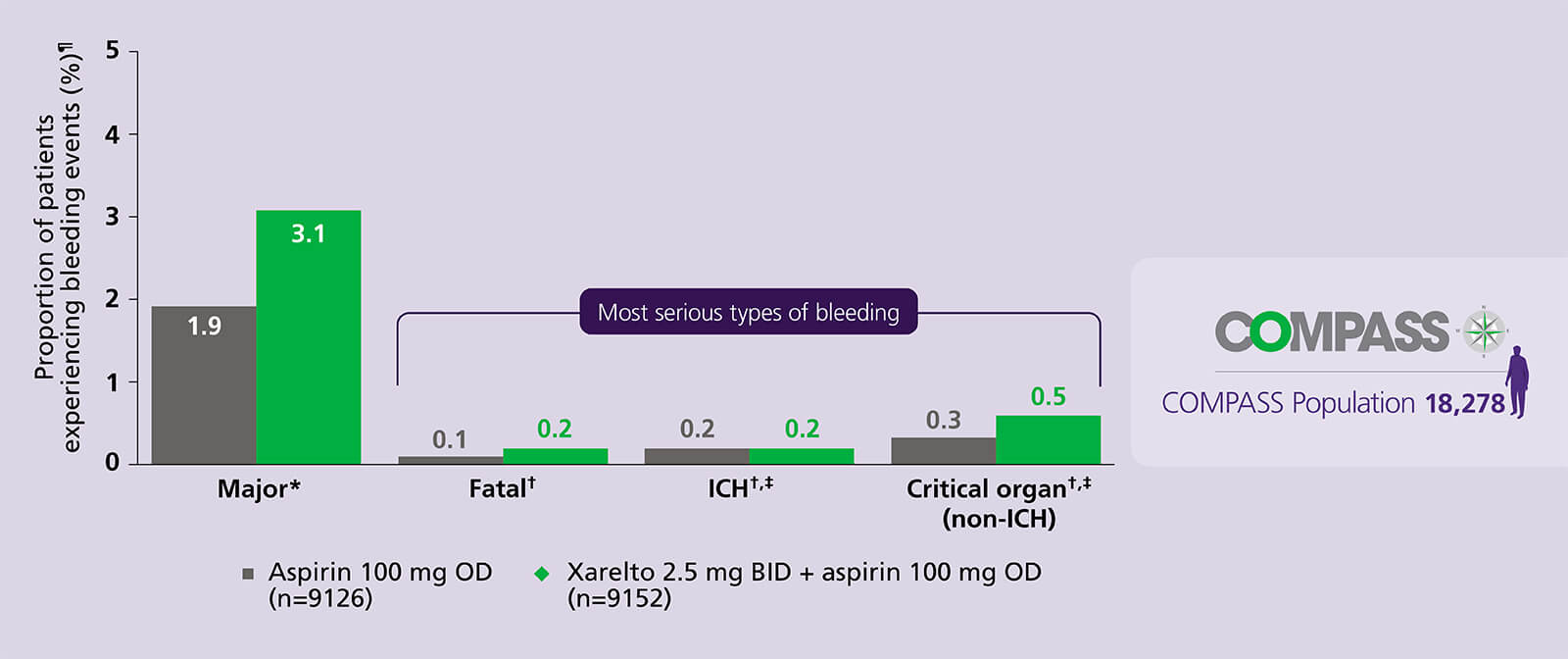
Protect your patients with a reassuring safety profile
Generally manageable bleeding, with no significant increase in the most serious types vs aspirin alone6
All about the COMPASS trial
The Xarelto COMPASS Trial: Rationale and results
PP-XAR-ALL-1790-1
References
- Xarelto (rivaroxaban) Summary of Product Characteristics. Xarelto (rivaroxaban) Summary of Product Characteristics. Return to content
- Cholesterol Treatment Trialists’ (CTT) Collaboration, et al. Lancet. 2015;385:1397–1405 Cholesterol Treatment Trialists’ (CTT) Collaboration, et al. Lancet. 2015;385:1397–1405 Return to content
- The Heart Outcomes Prevention Evaluation Study Investigators, et al. N Engl J Med. 2000;342:145–153 The Heart Outcomes Prevention Evaluation Study Investigators, et al. N Engl J Med. 2000;342:145–153 Return to content
- Marso SP, et al. N Engl J Med. 2016;375:311–322. Marso SP, et al. N Engl J Med. 2016;375:311–322. Return to content
- Bhatt DL, et al. J Am Coll Cardiol. 2007;49:1982–1928. Bhatt DL, et al. J Am Coll Cardiol. 2007;49:1982–1928. Return to content
- Eikelboom JW, et al. N Engl J Med. 2017;377:1319–1330. Eikelboom JW, et al. N Engl J Med. 2017;377:1319–1330. Return to content
- Connolly SJ, et al. Lancet. 2018;391:205–218. Connolly SJ, et al. Lancet. 2018;391:205–218. Return to content
- Anand SS, et al. Lancet. 2018;391:219–229. Anand SS, et al. Lancet. 2018;391:219–229. Return to content
- Bosch J, et al. Can J Cardiol. 2017;33:1027–1035. Bosch J, et al. Can J Cardiol. 2017;33:1027–1035. Return to content
- Schulman S, et al. J Thromb Haemost. 2005;3:692–694. Return to content
- Knuuti J, et al. Eur Heart J. 2019;ehz425. doi:10.1093/eurheartj/ehz425 Return to content
RELATED PODCAST
CAD or symptomatic PAD discussion