Clinical Studies – Targeting Acute and Chronic Thromboembolic Disorders
A Study of Flexible Dosage in Extended PE/DVT Treatment
EINSTEIN CHOICE:
A Study of Xarelto® with a Flexible Choice of Dosage for Extended Treatment of Recurrent VTE
Objective
- To compare the efficacy and safety profile of 20 mg OD or 10 mg OD Xarelto doses with aspirin for extended treatment of recurrent VTE , in patients who had been treated for 6–12 months with an anticoagulant therapy1
Study Design
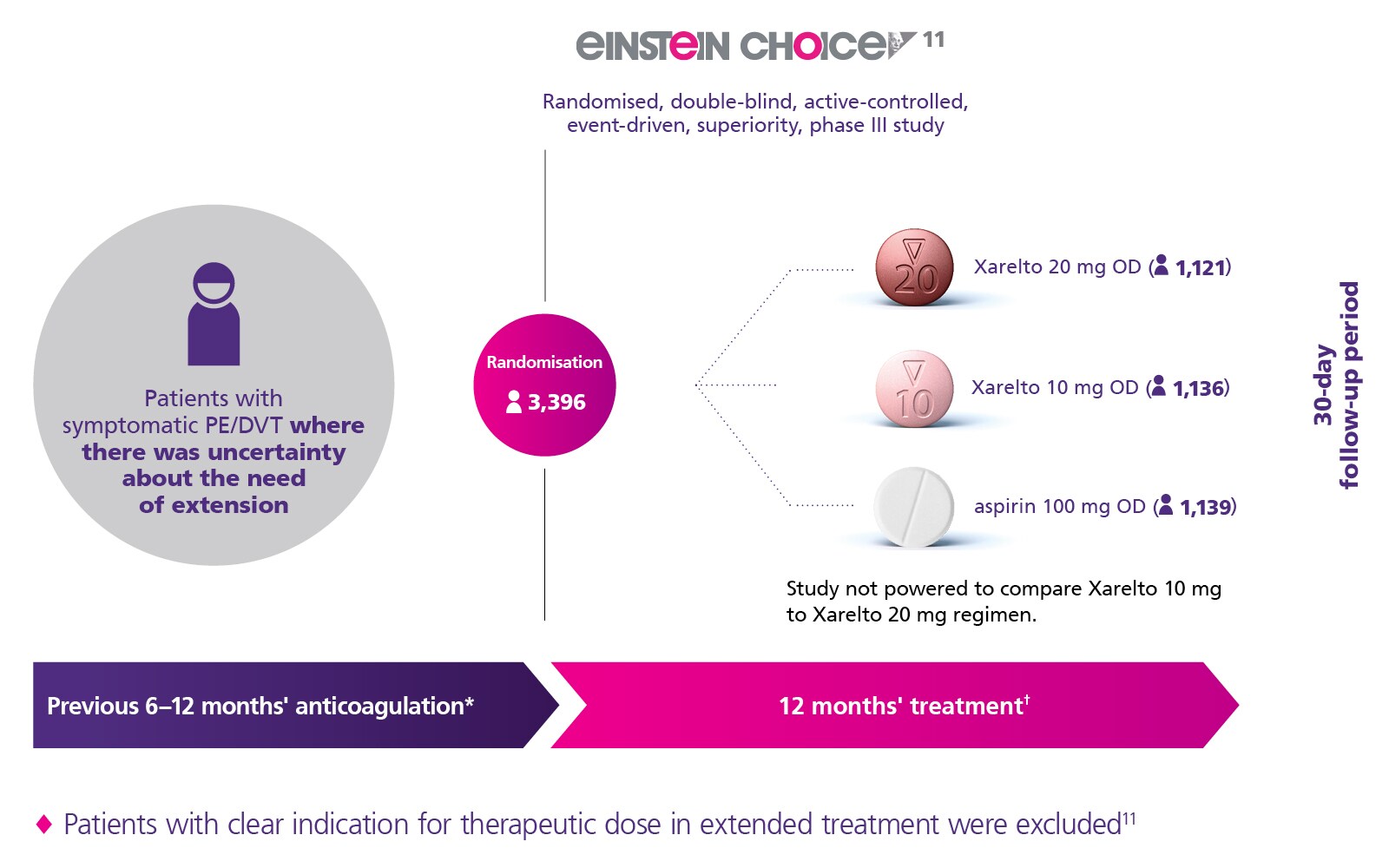
* ≤7 days of treatment interruption prior to randomisation. † Patients randomised after the requisite number of primary efficacy outcomes was reached were treated for ≥6 months.
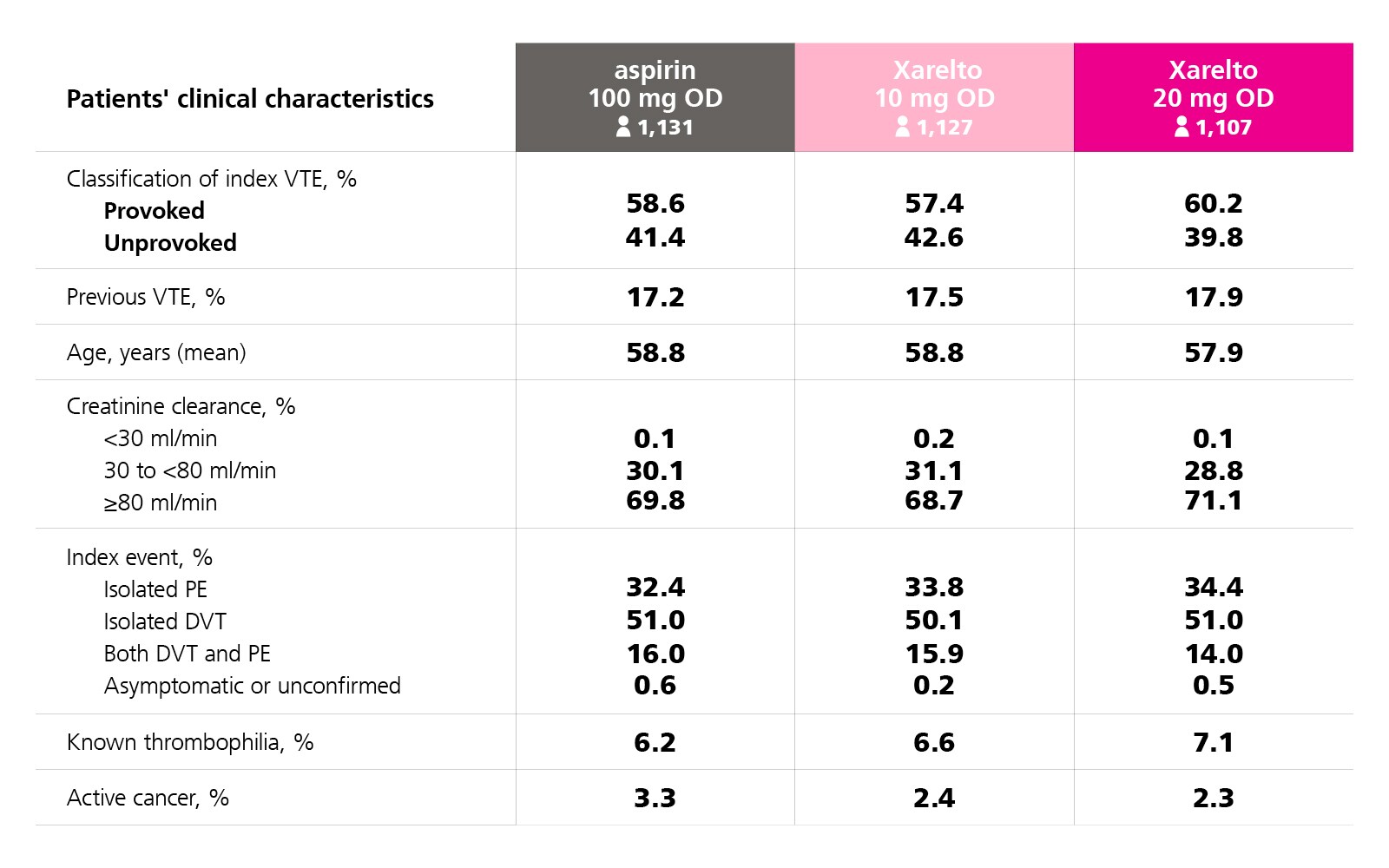
Patients’ Clinical Characteristics
Key Findings
- Efficacy
- Both 10 mg OD and 20 mg OD Xarelto doses significantly reduced the risk of recurrent VTE vs aspirin‡,1
- Safety
DVT , deep vein thrombosis ; OD, once daily; PE , pulmonary embolism; VTE , venous thromboembolism.
‡ For VTE extended treatment.
PP-XAR-ALL-1816-1
References
- Weitz JI, et al. N Engl J Med. 2017;376:1211–1222. Weitz JI, et al. N Engl J Med. 2017;376:1211–1222. Return to content







