Щоб відвідати глобальний вебсайт Ксарелто®, виберіть один із поданих нижче варіантів:
Цей вебсайт призначений для інформування міжнародної аудиторії за межами США, Великої Британії, Австрії та країн Південної Африки, у тому числі ПАР.
Якщо ви знаходитесь у США, натисніть на це посилання .
Labelings Menu
Ласкаво просимо на сайт «Ксарелто® | Байєр»
Згода користувача про отримання інформації про рецептурні лікарські засоби.
На даному сайті Компанія «Байєр» надає інформацію про рецептурні лікарські засоби, зокрема про їхню назву, характеристику, лікувальні властивості, можливу побічну дію, та іншу професійну спеціалізовану інформацію виключно професіоналам охорони здоров’я*. У випадку, якщо Ви не є професіоналом охорони здоров’я, проте, порушуючи ці умови, підписуєте дану угоду, Компанія «Байєр» не несе відповідальності за можливі негативні наслідки, що можуть виникнути в результаті самостійного використання Вами інформації з цього розділу сайту, без попередньої консультації з спеціалістом. Ви робите це самостійно та на власний розсуд, усвідомлюючи, що застосування рецептурних лікарських засобів можливо тільки після попередньої консультації з професіоналом охорони здоров’я. Інформація про рецептурні лікарські засоби Компанії «Байєр» надається виключно для ознайомлення з лікарськими засобами і не є рекламою, та не є керівництвом для самостійної діагностики або лікування, а також, не може бути використана в якості медичних порад або рекомендацій. Компанія «Байєр» не несе відповідальності за можливу шкоду, спричинену Вашому здоров’ю у разі самостійного лікування, що проводиться на базі використання рецептурних лікарських засобів (без попередньої консультації зі спеціалістом). *Професіонал сфери охорони здоров’я - особа, яка має закінчену медичну або фармацевтичну освіту та відповідну кваліфікацію, працює у сфері охорони здоров'я та в силу своїх службових обов'язків чи повноважень може впливати на призначення та/або придбання лікарських засобів.
Цим я підтверджую, що є спеціалістом охорони здоров’я та підтримую угоду про отримання інформації про рецептурні лікарські засоби.
Цей вебсайт призначений для інформування міжнародної аудиторії за межами США,
Великої Британії, Австрії та країн Південної Африки, у тому числі ПАР.
Якщо ви знаходитесь у США, натисніть на це посилання.
Clinical Studies – Targeting Acute and Chronic Thromboembolic Disorders
EINSTEIN programme
Sign up for more content
Sign up for more content
Register to gain access to personalised information regarding Brand, the indications your are treating regularly, dosing guidelines and resources such as Major Studies, Patient Information and short videos curated to your special interest.
Treatment of Venous Thromboembolism:
the EINSTEIN Programme
The EINSTEIN clinical trial programme comprises four randomised Phase 3 studies of rivaroxaban for the treatment of VTE and the long-term prevention of recurrent VTE. It is the only clinical programme that has investigated a new oral anticoagulant for the treatment of acute DVT and treatment for acute PE in separate trials. It also comprises of the first completed trial of an oral anticoagulant in children, the largest study to date in children with VTE.
- EINSTEIN DVT: rivaroxaban versus enoxaparin plus a vitamin K antagonist (VKA) in the treatment of acute DVT without symptomatic PE
- EINSTEIN PE: rivaroxaban versus enoxaparin plus a VKA in the treatment of acute PE with or without symptomatic DVT
- EINSTEIN EXT: rivaroxaban versus placebo in the long-term prevention of recurrent, symptomatic VTE in patients who had already received 6–12 months of anticoagulant treatment for DVT or PE
- EINSTEIN JUNIOR: rivaroxaban vs continuation of heparin/VKA in the prevention of recurrent VTE in children aged 0–17 years
EINSTEIN DVT design5
Single-drug therapy with oral rivaroxaban versus dual-drug therapy with subcutaneous enoxaparin and oral VKA
Acute DVT is a common disorder. The current standard of care is dual-drug therapy with a parenteral agent (such as unfractionated heparin, low molecular weight heparin or fondaparinux) plus a VKA followed by international normalised ratio (INR)-adjusted VKA alone.1 Although short-term treatment with the current standard of care is effective, management of acute DVT with VKA therapy in the outpatient setting remains challenging. Many of these challenges relate to the limitations of VKA therapy, which include:2
- Narrow therapeutic window
- Requirement for regular coagulation monitoring and dose adjustment
- Multiple food and drug interactions
- Lifestyle limitations
Rivaroxaban, as a newer oral anticoagulant, overcomes many of these limitations. It has a rapid onset of action and so does not require bridging therapy with parenteral agents. It does not require routine coagulation monitoring and has fewer interactions with commonly prescribed drugs; therefore, rivaroxaban provides a convenient, simplified treatment option for patients, with a consequent positive impact on daily life.
Objective
The main objective of EINSTEIN DVT was to determine whether a single-drug approach with oral rivaroxaban was at least as effective as (non-inferior to) dual-drug therapy with enoxaparin/VKA and to compare the safety of these two approaches for the treatment of patients with acute symptomatic DVT without symptomatic PE.
Study design
EINSTEIN DVT was a randomised, open-label, event-driven, non-inferiority study of 3449 patients with a predefined treatment duration of 3, 6 or 12 months. The patients were randomly assigned to receive one of the following regimens:
- Oral rivaroxaban 15 mg twice daily for 3 weeks followed by 20 mg once daily for the remaining treatment period
- Subcutaneous, body weight-adjusted enoxaparin twice daily for at least 5 days plus a VKA, followed by dose-adjusted VKA only (target INR of 2.0–3.0) for the remaining treatment period
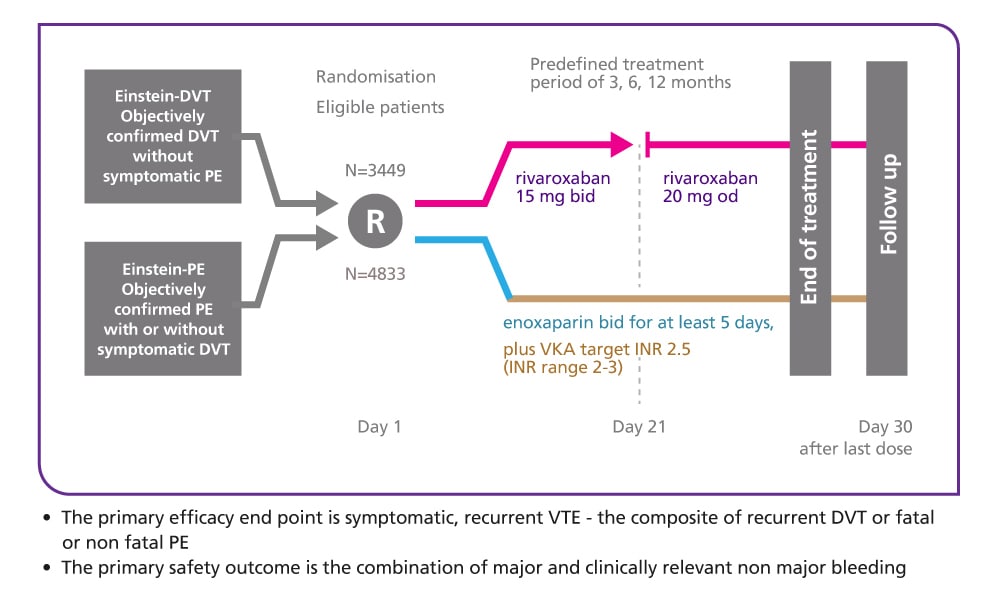
Endpoints
- The primary efficacy endpoint was symptomatic, recurrent VTE – the composite of recurrent DVT or fatal or non-fatal PE
- The principal safety outcome was clinically relevant bleeding, defined as the composite of major bleeding or non-major clinically relevant bleeding; major bleeding was defined as overt bleeding associated with any of the following:
- A decrease in haemoglobin of 2 g/dl or more
- A transfusion of two or more units of packed red blood cells or whole blood
- Occurrence at a critical site, such as intracranial or retroperitoneal
- Death
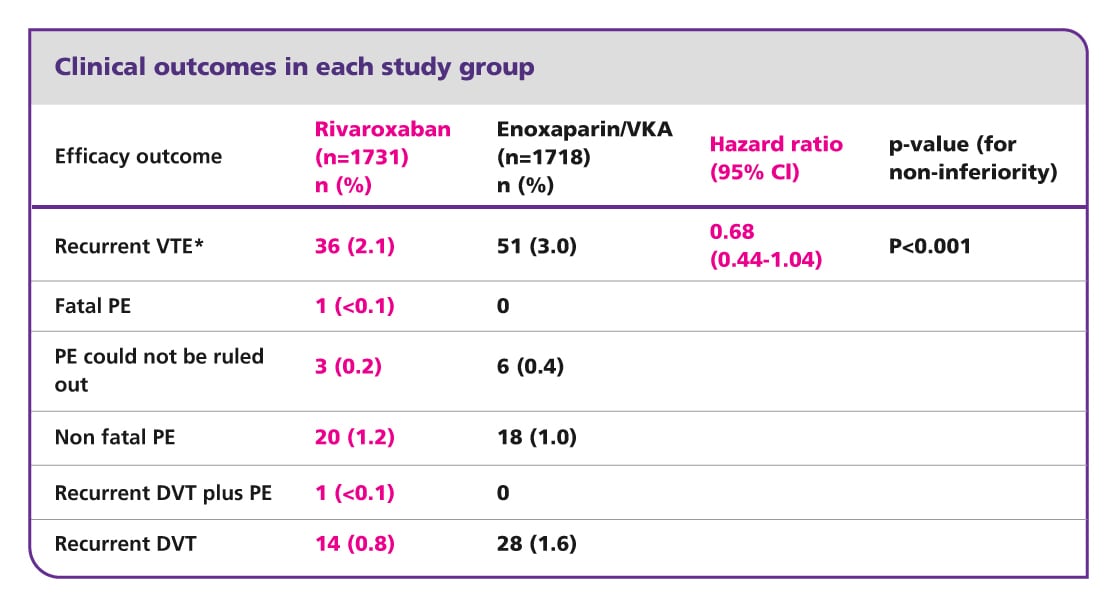
EINSTEIN DVT results5
Efficacy and safety of rivaroxaban in the treatment of deep vein thrombosis
EINSTEIN DVT met its primary efficacy endpoint and showed that a single-drug approach with oral rivaroxaban was at least as effective as (non-inferior to) conventional dual-drug therapy (subcutaneous enoxaparin plus vitamin K antagonist [VKA] followed by VKA alone), with a similar safety profile, for the treatment of acute, symptomatic deep vein thrombosis (DVT).
There was an improvement in the net clinical benefit for rivaroxaban compared with enoxaparin/VKA, achieved via a reduction in recurrent venous thromboembolism and a numerically lower incidence of major bleeding.
In conclusion, EINSTEIN DVT demonstrated that a fixed-dose regimen of oral rivaroxaban offers an effective and convenient single-drug alternative, with reassuring safety, to the current standard of care of dual-drug therapy for the treatment of acute DVT.
Patient demographics
Patients were well matched in both study arms, with similar demographic and clinical characteristics:
- Mean age: ~56 years
- ~57% were male
- ~83% had a body weight of >50 to 100 kg
- ~69% had creatinine clearance ≥80 ml/min, with a further 23% classified as having mild renal impairment (creatinine clearance 50–79 ml/min)
- ~19% had experienced a previous venous thromboembolic event before the acute, symptomatic DVT that qualified them for inclusion in the study
Primary efficacy endpoint: single-drug therapy with rivaroxaban was non-inferior to dual-drug therapy with enoxaparin plus VKA for the treatment of acute DVT
The primary efficacy outcome occurred in 36/1731 (2.1%) patients treated with rivaroxaban, compared with 51/1718 (3.0%) patients treated with enoxaparin/VKA (p<0.001 for non-inferiority);
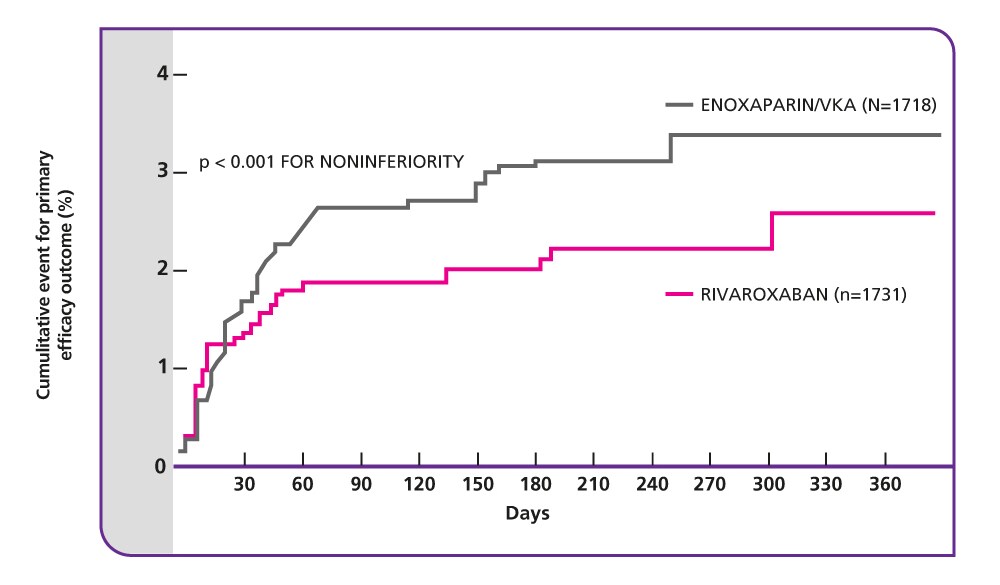
Source: The Einstein Investigators (2010).
CI, confidence interval; DVT, deep vein thrombosis; PE, pulmonary embolism; VKA, vitamin K antagonist; VTE, venous thromboembolism.
*Composite of DVT or non-fatal or fatal PE.
Safety outcome: similar rates of bleeding with rivaroxaban and enoxaparin/VKA
Rivaroxaban was well-tolerated by patients in EINSTEIN DVT, and the rate of major or non-major clinically relevant bleeding was similar to that for enoxaparin plus VKA followed by VKA alone.
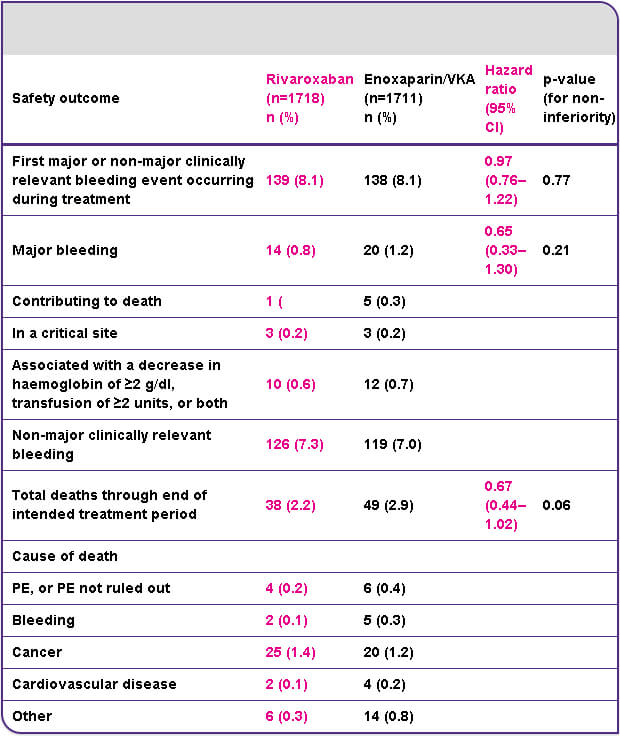
CI, confidence interval; PE, pulmonary embolism; VKA, vitamin K antagonist.
Rivaroxaban provides superior net clinical benefit
A key secondary endpoint in the study was net clinical benefit; defined as the composite of the primary efficacy endpoint and major bleeding.
- The net clinical benefit outcome occurred in 51/1731 (2.9%) patients who received rivaroxaban compared with 73/1718 (4.2%) patients who received enoxaparin/VKA (hazard ratio 0.67; 95% confidence interval 0.47–0.95; p=0.03)
Adverse event rates were similar in the two treatment groups during the treatment period
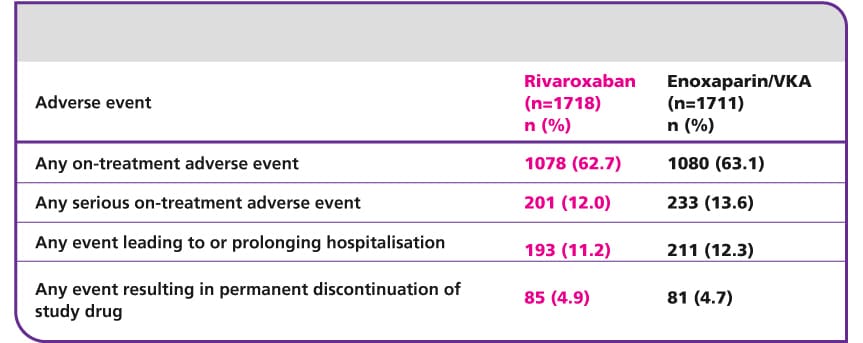
CI, confidence interval; DVT, deep vein thrombosis; INR, international normalised ratio; PE, pulmonary embolism; VKA, vitamin K antagonis; VTE, venous thromboembolism.
EINSTEIN PE design6
Single-drug therapy with oral rivaroxaban versus dual-drug therapy with subcutaneous enoxaparin and oral VKA
PE is the most severe complication of DVT. It is associated with significant mortality and is often undiagnosed.3 Up to 26% of patients with untreated, clinically diagnosed PE die within 2 weeks.4 The current standard of care is initial parenteral therapy (with unfractionated heparin, low molecular weight heparin or fondaparinux) plus a VKA, followed by long-term management with INR-adjusted VKA alone.5 Short-term treatment with conventional anticoagulant therapy is effective, but long-term management with VKA therapy is challenging. Many of these challenges relate to the limitations of VKA therapy, which include change to colon.2
- Narrow therapeutic window
- Requirement for frequent coagulation monitoring and dose adjustments
- Multiple food and drug interactions
- Lifestyle limitations
Rivaroxaban, as a newer oral anticoagulant, overcomes many of these limitations. It has a rapid onset of action and so does not require bridging therapy with parenteral agents. It does not require routine coagulation monitoring and has fewer interactions with commonly prescribed drugs; therefore, rivaroxaban provides a convenient, simplified treatment option for patients, with a consequent positive impact on daily life.
Objective
The objective of EINSTEIN PE was to determine whether a single-drug approach with oral rivaroxaban is at least as effective as (non-inferior to) dual-drug therapy with enoxaparin/VKA and to compare the safety of these two approaches in the treatment of patients with confirmed acute symptomatic PE with or without symptomatic DVT.
Study design
EINSTEIN PE was a randomised, open-label, event-driven, non-inferiority study of 4833 patients with a predefined treatment duration of 3, 6 or 12 months. The patients were randomly assigned to receive one of the following regimens:
- Oral rivaroxaban 15 mg twice daily for 3 weeks followed by 20 mg once daily for the remaining treatment period
- Subcutaneous body weight-adjusted enoxaparin twice daily for at least 5 days plus a VKA, followed by dose-adjusted VKA only (target INR of 2.0–3.0) for the remaining treatment period
Endpoints
- The primary efficacy endpoint was symptomatic, recurrent VTE – the composite of recurrent DVT or fatal or non-fatal PE
- The principal safety outcome was the combination of major and non-major clinically relevant bleeding; major bleeding was defined as overt bleeding associated with any of the following:
- A decrease in haemoglobin of 2 g/dl or more
- A transfusion of two or more units of packed red blood cells or whole blood
- Occurrence at a critical site, such as intracranial or retroperitoneal
- Death

EINSTEIN PE results6
Efficacy and safety of rivaroxaban in the treatment of pulmonary embolism
EINSTEIN PE demonstrated that oral rivaroxaban was at least as effective as (non-inferior to) standard dual-drug therapy with subcutaneous enoxaparin plus VKA followed by VKA alone for the treatment of acute, symptomatic PE with or without symptomatic DVT. It also demonstrated that rivaroxaban had a similar rate of clinically relevant bleeding to standard therapy but significantly reduced the incidence of major bleeding.
In conclusion, EINSTEIN PE demonstrates that oral rivaroxaban is a simple and effective, single-drug solution for the treatment of acute PE with or without symptomatic DVT, with an improved benefit–risk profile compared with the standard of care.
Patient demographics
Patients were well matched in both study arms, with similar demographic and clinical characteristics:
- Mean age: ~58 years
- ~53% were male
- ~83% had a body weight of >50 to 100 kg
- ~66% had creatinine clearance ≥80 ml/min, with a further 25% classified as having mild renal impairment (creatinine clearance 50–79 ml/min)
- ~24% had extensive PE (involving multiple lobes and >25% of entire pulmonary vasculature)
- ~25% had concurrent symptomatic DVT
- ~19% had experienced a previous venous thromboembolic event before the acute, symptomatic and objectively confirmed PE that qualified them for inclusion in the study
Primary efficacy endpoint: single-drug therapy with rivaroxaban was non-inferior to dual-drug therapy with enoxaparin plus VKA for the treatment of acute PE
The primary efficacy outcome (composite of non-fatal or fatal PE or DVT) occurred in 50/2419 (2.1%) patients treated with rivaroxaban, compared with 44/2413 (1.8%) patients treated with standard therapy (p=0.003 for non-inferiority).
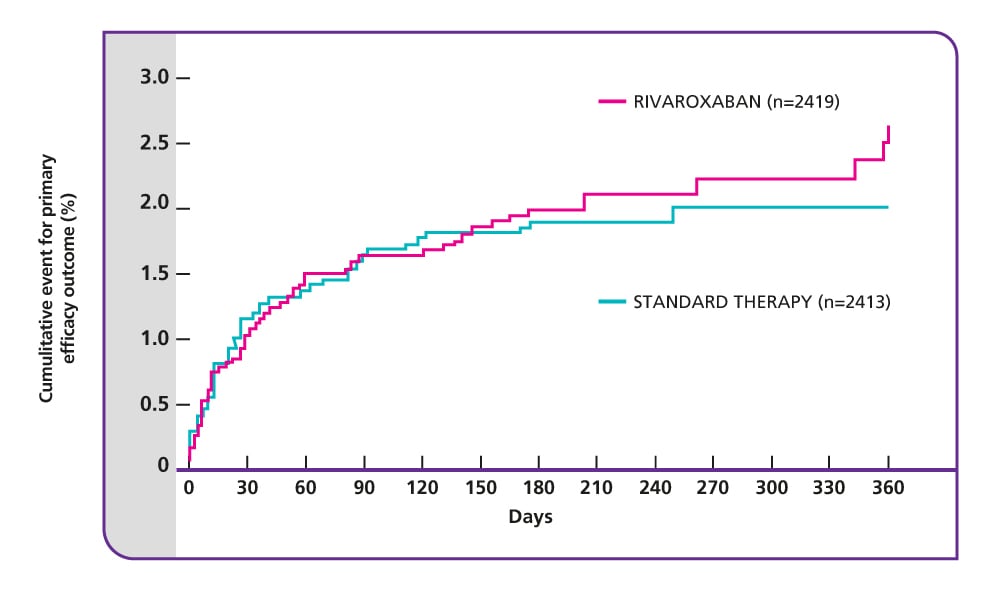
Source: The EINSTEIN PE Investigators. (2012). VKA, vitamin K antagonist.
Clinical outcomes in each study group
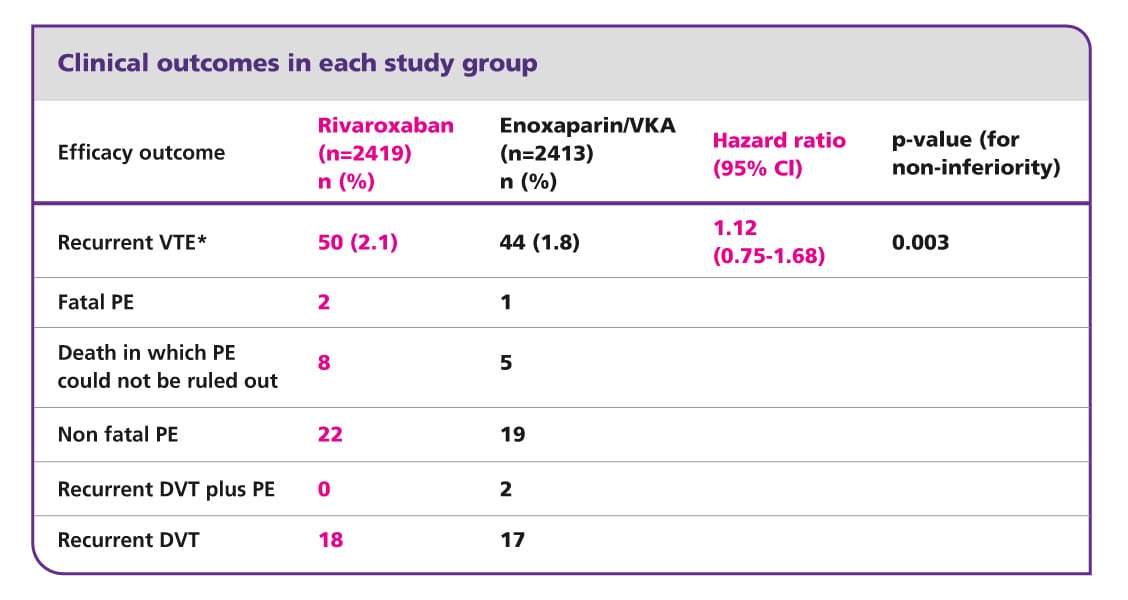
Source: The EINSTEIN PE Investigators. (2012). VKA, vitamin K antagonist.
*Composite of non-fatal or fatal PE.
CI, confidence interval; DVT, deep vein thrombosis; PE, pulmonary embolism; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Rivaroxaban had similar rates of clinically relevant bleeding compared with standard therapy but reduced major bleeding by 51%
In EINSTEIN PE, the rate of major or non-major clinically relevant bleeding in patients receiving rivaroxaban was similar to that for standard therapy. Importantly, major bleeding was significantly reduced in patients receiving rivaroxaban compared with those receiving standard therapy (p=0.003) and the rates of critical site bleeding were markedly lower for rivaroxaban.
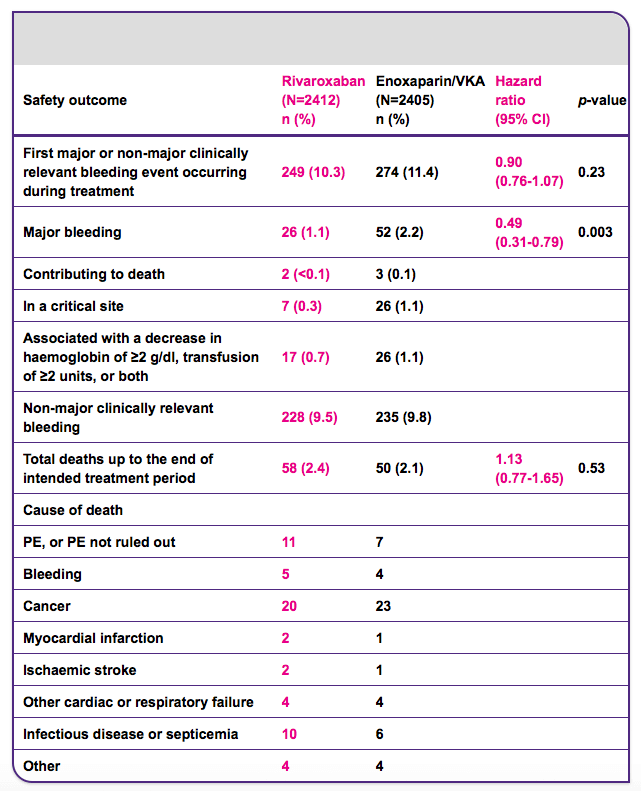
CI, confidence interval; PE, pulmonary embolism; VKA, vitamin K antagonist.
Rivaroxaban provides comparable net clinical benefit to standard therapy
Net clinical benefit, defined as the composite of the primary efficacy endpoint and major bleeding, was a key secondary endpoint in EINSTEIN PE.
In EINSTEIN PE, rivaroxaban had a similar net clinical benefit compared with enoxaparin plus VKA followed by VKA alone, with numerically fewer events.
- The net clinical benefit outcome occurred in 83/2419 (3.4%) patients who received rivaroxaban compared with 96/2413 (4.0%) patients who received standard therapy (hazard ratio 0.85; 95% confidence interval 0.63–1.14; p=0.28)
Adverse event rates were similar in the two treatment groups during the treatment period
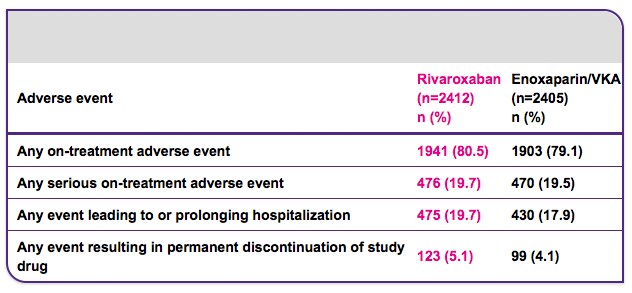
VKA, vitamin.
EINSTEIN EXT design5
The long-term prevention of recurrent, symptomatic VTE
Effective treatment of an acute venous thromboembolic event reduces the risk of recurrence dramatically, from an estimated 25% to approximately 3% during the first 6–12 months. However, the optimal duration of anticoagulation therapy after an acute event remains a matter of debate. Treatment with VKAs beyond 1 year is associated with an annual risk of major bleeding of 1–2%,5 and clinicians must balance the long-term risks of recurrent VTE if anticoagulation is halted versus the risk of bleeding with further therapy and the healthcare burden of managing this therapy. Long-term management with VKA therapy is challenging and many of these challenges relate to limitations of VKA therapy, which include:2
- Narrow therapeutic window
- Requirement for frequent coagulation monitoring and dose adjustments
- Multiple food and drug interactions
- Lifestyle limitations
Rivaroxaban, as a newer oral anticoagulant, simplifies DVT treatment and prevention of recurrent DVT and PE. It does not require routine coagulation monitoring and has fewer interactions with commonly prescribed drugs; therefore, rivaroxaban provides a convenient, simplified treatment option for patients, with a consequent positive impact on daily life.
Objective
The main efficacy objective of the EINSTEIN EXT study was to demonstrate that fixed-dose oral rivaroxaban (20 mg once daily) was superior to placebo in the long-term secondary prevention of recurrent VTE in patients with symptomatic DVT or PE who had already completed 6–12 months of VTE treatment with either a VKA or rivaroxaban and in whom there was clinical uncertainty as to whether to continue anticoagulation. Safety was also evaluated.
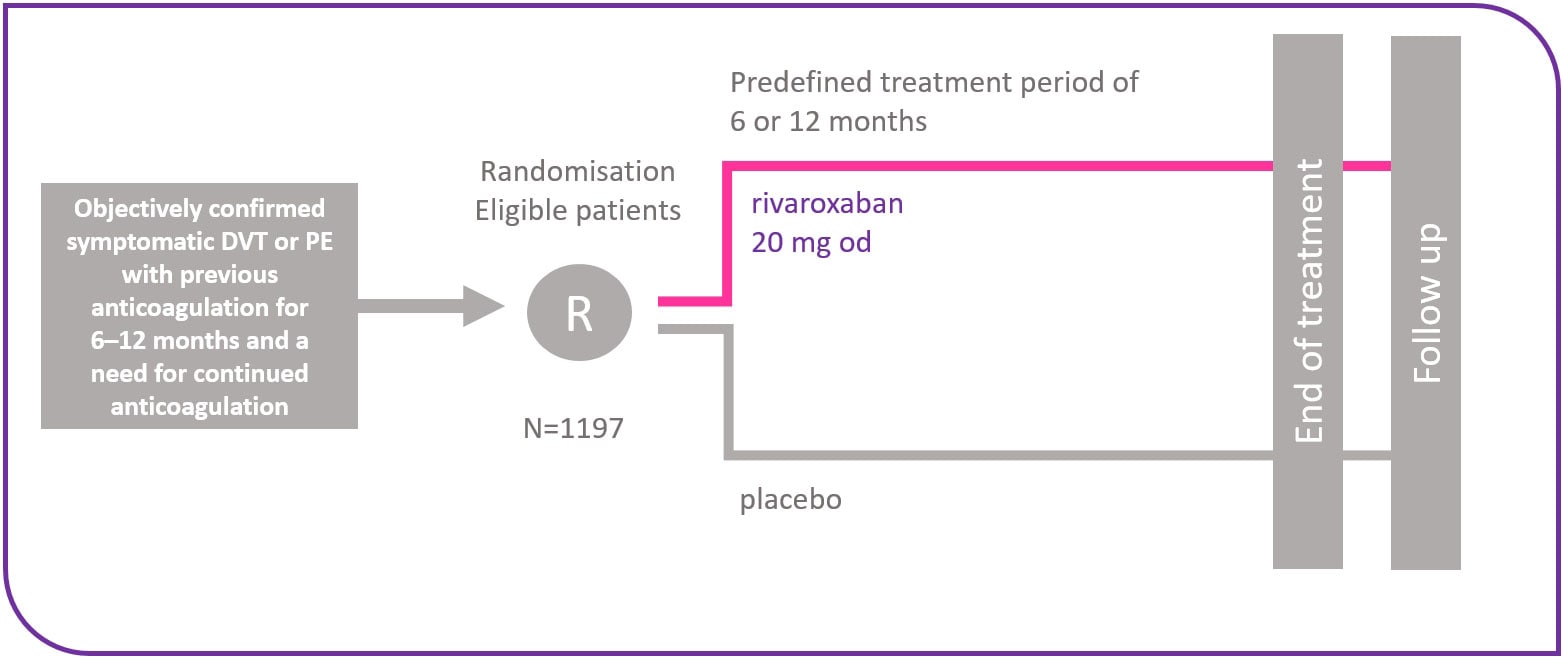
Study design
EINSTEIN EXT was a double-blind, randomised, placebo-controlled, event-driven superiority study of 1197 patients who were at clinical equipoise regarding the need for continued VKA therapy. Patients who had completed the EINSTEIN DVT or EINSTEIN PE studies could be recruited into EINSTEIN EXT. In addition, patients who had not participated in these studies were also enrolled, if they had received treatment with a VKA up to randomised after an initial diagnosis of DVT or PE. The patients were randomly assigned to one of the following regimens:
- Rivaroxaban 20 mg tablet once daily
- Placebo once daily
In both arms, the treatment duration was either 6 or 12 months.
Endpoints
- The primary efficacy endpoint was symptomatic, recurrent VTE – the composite of recurrent DVT or fatal or non-fatal PE
- The principal safety outcome was major bleeding, defined as overt bleeding associated with any of the following:
- A decrease in haemoglobin of 2 g/dl or more
- A transfusion of two or more units of packed red blood cells or whole blood
- Occurrence at a critical site: intracranial or retroperitoneal
- Death
EINSTEIN EXT results5
Efficacy and safety of rivaroxaban for prevention of recurrent venous thromboembolism
EINSTEIN EXT met its primary efficacy endpoint and showed that extended-duration rivaroxaban (20 mg once daily for 6–12 months) was superior – compared with placebo – for prevention of recurrent VTE in patients who had previously completed 6–12 months of treatment for VTE with either rivaroxaban or a VKA.
Rivaroxaban showed a favourable benefit–risk profile, as demonstrated by a significant improvement in the net clinical benefit, which was defined as the composite of the primary efficacy outcome and major bleeding.
In conclusion, EINSTEIN EXT demonstrated that rivaroxaban offers effective long-term prevention of recurrent VTE with reassuring safety.
Patient demographics
At study entry, patients in the rivaroxaban and placebo groups had similar demographic and clinical characteristics:
- Mean age: ~58 years
- ~58% were male
- ~82% had a body weight of >50 to 100 kg
- ~62% had creatinine clearance ≥80 ml/min, with a further 22% classified as having mild renal impairment (creatinine clearance 50–79 ml/min)
- ~16% had experienced a previous venous thromboembolic event before the deep vein thrombosis (DVT) or pulmonary embolism (PE) that qualified them for inclusion in the study
Primary efficacy endpoint: 82% relative risk reduction with rivaroxaban
The EINSTEIN EXT study included patients about whom there is clinical uncertainty whether to continue or stop anticoagulant therapy after initial prophylaxis for 6–12 months with a VKA or rivaroxaban. This study showed that anticoagulation with rivaroxaban for a further 6 or 12 months was superior to placebo for the long-term prevention of recurrent symptomatic VTE. It also showed that there is still a sustained risk of recurrence in patients with symptomatic DVT or PE after the initial anticoagulant therapy for 6–12 months.
- To prevent one venous thromboembolic event, only 15 patients need to be treated with rivaroxaban1
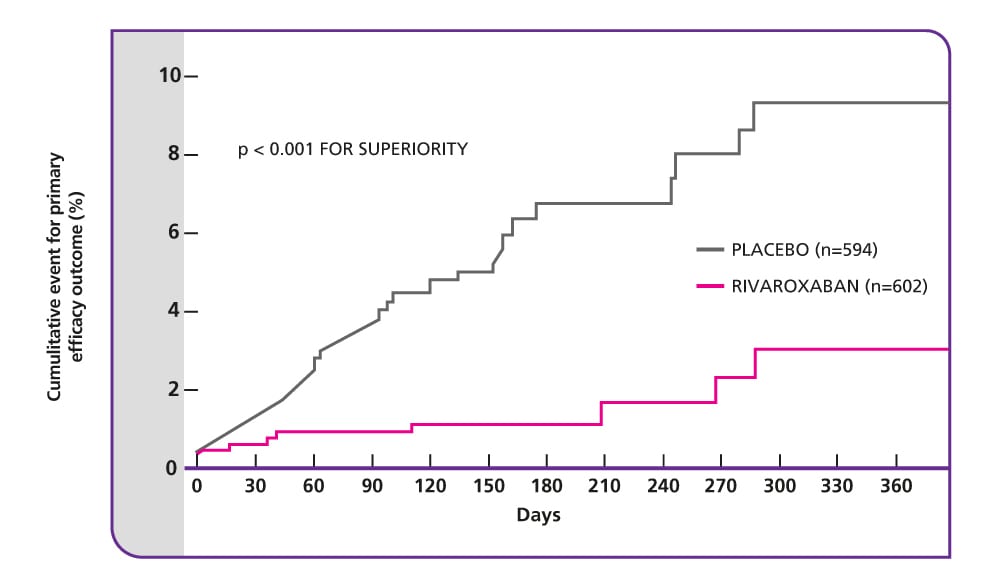
Source: The EINSTEIN Investigators. (2010).
Composite of non-fatal or fatal PE or DVT.
CI, confidence interval; DVT, deep vein thrombosis; PE, pulmonary embolism; VKA, vitamin K antagonist; VTE, venous thromboembolism.
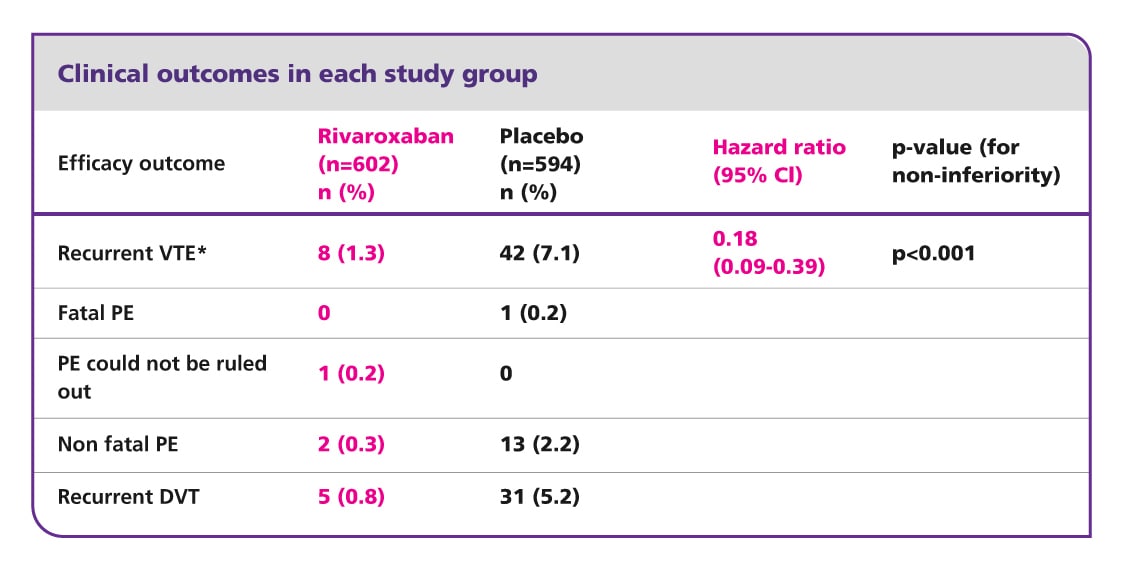
Safety outcome: incidence of major bleeding was low in the rivaroxaban arm
The primary safety outcome was major bleeding, which was low and occurred in 4/598 (0.7%) patients in the rivaroxaban group and in none of the patients in the placebo group resulting in a non-significant difference (p=0.11). All cases of major bleeding in the rivaroxaban arm did not occur in a critical site, but were associated with a fall in haemoglobin of ≥2 g/dl, transfusion of ≥2 units, or both. The most common non-major clinically relevant bleeding events in the rivaroxaban arm were haematuria (9/32), epistaxis (8/32) and rectal bleeding (7/32); one event of gastrointestinal bleeding occurred (1/32).
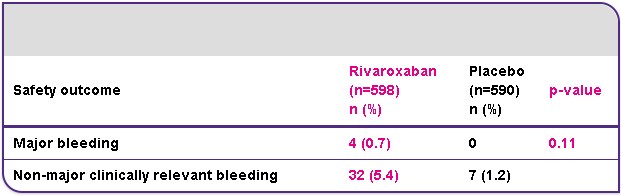
Rivaroxaban provides superior net clinical benefit
Compared with placebo, rivaroxaban provided a significantly improved net clinical benefit; a prespecified secondary endpoint defined as the composite of the primary efficacy endpoint and major bleeding. This outcome occurred in 12/602 (2.0%) patients receiving rivaroxaban and in 42/594 (7.1%) patients receiving placebo (hazard ratio 0.28; 95% CI 0.15–0.53; p<0.001).
EINSTEIN Junior design7
Bodyweight adjusted rivaroxaban in a 20 mg-equivalent dose versus the standard of care* in children
* Following 5–9 days of LMWH/unfractionated heparin/fondaparinux, patients either continued treatment with or were switched to a VKA.7
EINSTEIN EXT met its primary efficacy endpoint and showed that extended-duration rivaroxaban (20 mg once daily for 6–12 months) was superior – compared with placebo – for prevention of recurrent VTE in patients who had previously completed 6–12 months of treatment for VTE with either rivaroxaban or a VKA.
Rivaroxaban showed a favourable benefit–risk profile, as demonstrated by a significant improvement in the net clinical benefit, which was defined as the composite of the primary efficacy outcome and major bleeding.
In conclusion, EINSTEIN EXT demonstrated that rivaroxaban offers effective long-term prevention of recurrent VTE with reassuring safety.
Patient demographics
At study entry, patients in the rivaroxaban and placebo groups had similar demographic and clinical characteristics:
- Mean age: ~58 years
- ~58% were male
- ~82% had a body weight of >50 to 100 kg
- ~62% had creatinine clearance ≥80 ml/min, with a further 22% classified as having mild renal impairment (creatinine clearance 50–79 ml/min)
- ~16% had experienced a previous venous thromboembolic event before the deep vein thrombosis (DVT) or pulmonary embolism (PE) that qualified them for inclusion in the study
Primary efficacy endpoint: 82% relative risk reduction with rivaroxaban
The EINSTEIN EXT study included patients about whom there is clinical uncertainty whether to continue or stop anticoagulant therapy after initial prophylaxis for 6–12 months with a VKA or rivaroxaban. This study showed that anticoagulation with rivaroxaban for a further 6 or 12 months was superior to placebo for the long-term prevention of recurrent symptomatic VTE. It also showed that there is still a sustained risk of recurrence in patients with symptomatic DVT or PE after the initial anticoagulant therapy for 6–12 months.
- To prevent one venous thromboembolic event, only 15 patients need to be treated with rivaroxaban1

Source: The EINSTEIN Investigators. (2010).

Composite of non-fatal or fatal PE or DVT.
CI, confidence interval; DVT, deep vein thrombosis; PE, pulmonary embolism; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Safety outcome: incidence of major bleeding was low in the rivaroxaban arm
The primary safety outcome was major bleeding, which was low and occurred in 4/598 (0.7%) patients in the rivaroxaban group and in none of the patients in the placebo group resulting in a non-significant difference (p=0.11). All cases of major bleeding in the rivaroxaban arm did not occur in a critical site, but were associated with a fall in haemoglobin of ≥2 g/dl, transfusion of ≥2 units, or both. The most common non-major clinically relevant bleeding events in the rivaroxaban arm were haematuria (9/32), epistaxis (8/32) and rectal bleeding (7/32); one event of gastrointestinal bleeding occurred (1/32).

Rivaroxaban provides superior net clinical benefit
Compared with placebo, rivaroxaban provided a significantly improved net clinical benefit; a prespecified secondary endpoint defined as the composite of the primary efficacy endpoint and major bleeding. This outcome occurred in 12/602 (2.0%) patients receiving rivaroxaban and in 42/594 (7.1%) patients receiving placebo (hazard ratio 0.28; 95% CI 0.15–0.53; p<0.001).
EINSTEIN Junior results7
Efficacy and safety of rivaroxaban for the prevention of recurrent venous thromboembolism
EINSTEIN EXT met its primary efficacy endpoint and showed that extended-duration rivaroxaban (20 mg once daily for 6–12 months) was superior – compared with placebo – for prevention of recurrent VTE in patients who had previously completed 6–12 months of treatment for VTE with either rivaroxaban or a VKA.
Rivaroxaban showed a favourable benefit–risk profile, as demonstrated by a significant improvement in the net clinical benefit, which was defined as the composite of the primary efficacy outcome and major bleeding.
In conclusion, EINSTEIN EXT demonstrated that rivaroxaban offers effective long-term prevention of recurrent VTE with reassuring safety.
Patient demographics
At study entry, patients in the rivaroxaban and placebo groups had similar demographic and clinical characteristics:
- Mean age: ~58 years
- ~58% were male
- ~82% had a body weight of >50 to 100 kg
- ~62% had creatinine clearance ≥80 ml/min, with a further 22% classified as having mild renal impairment (creatinine clearance 50–79 ml/min)
- ~16% had experienced a previous venous thromboembolic event before the deep vein thrombosis (DVT) or pulmonary embolism (PE) that qualified them for inclusion in the study
Primary efficacy endpoint: 82% relative risk reduction with rivaroxaban
The EINSTEIN EXT study included patients about whom there is clinical uncertainty whether to continue or stop anticoagulant therapy after initial prophylaxis for 6–12 months with a VKA or rivaroxaban. This study showed that anticoagulation with rivaroxaban for a further 6 or 12 months was superior to placebo for the long-term prevention of recurrent symptomatic VTE. It also showed that there is still a sustained risk of recurrence in patients with symptomatic DVT or PE after the initial anticoagulant therapy for 6–12 months.
- To prevent one venous thromboembolic event, only 15 patients need to be treated with rivaroxaban1

Source: The EINSTEIN Investigators. (2010).

Composite of non-fatal or fatal PE or DVT.
CI, confidence interval; DVT, deep vein thrombosis; PE, pulmonary embolism; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Safety outcome: incidence of major bleeding was low in the rivaroxaban arm
The primary safety outcome was major bleeding, which was low and occurred in 4/598 (0.7%) patients in the rivaroxaban group and in none of the patients in the placebo group resulting in a non-significant difference (p=0.11). All cases of major bleeding in the rivaroxaban arm did not occur in a critical site, but were associated with a fall in haemoglobin of ≥2 g/dl, transfusion of ≥2 units, or both. The most common non-major clinically relevant bleeding events in the rivaroxaban arm were haematuria (9/32), epistaxis (8/32) and rectal bleeding (7/32); one event of gastrointestinal bleeding occurred (1/32).

Rivaroxaban provides superior net clinical benefit
Compared with placebo, rivaroxaban provided a significantly improved net clinical benefit; a prespecified secondary endpoint defined as the composite of the primary efficacy endpoint and major bleeding. This outcome occurred in 12/602 (2.0%) patients receiving rivaroxaban and in 42/594 (7.1%) patients receiving placebo (hazard ratio 0.28; 95% CI 0.15–0.53; p<0.001).
PP-XAR-ALL-1822-1
References
- Steiff MB et al. J Thromb Thrombolysis. 2016;41:32-67. Return to content
- Ageno W, et al. Chest. 2012;141:e44S–e88S. Return to content
- Cohen AT, et al. Thromb Haemost. 2007;98:756–764. Return to content
- Kearon C. Circulation. 2003;107(23 suppl 1):I22–I30. Return to content
- The EINSTEIN Investigators. N Engl J Med. 2010;363:2499-510. Return to content
- The EINSTEIN-PE Investigators. N Engl J Med. 2012;366:1287-1297. Return to content
- Male C et al. Lancet Haematol 2020;7:e18–e27. Return to content
© Байєр Україна
Інформація, розміщена на даному вебсайті, призначена виключно для спеціалістів у сфері охорони здоров’я і лише з ознайомчою метою.
Дата останнього оновлення сайту: грудень 2024 року
PP-XAR-UA-0858-1_ 18 Dec 2024







