Clinical Studies – Targeting Acute and Chronic Thromboembolic Disorders
SELECT-D: A randomised control trial of Xarelto vs dalteparin in patients with active cancer
Background1
- The risk of recurrent VTE is increased at least two-fold in patients with cancer compared to those without
- For more than a decade, subcutaneous low-molecular weight heparin (LMWH) has been the standard of care
Objective1
- To assess if an oral factor Xa inhibitor, Xarelto, would offer an alternative treatment for VTE in patients with cancer
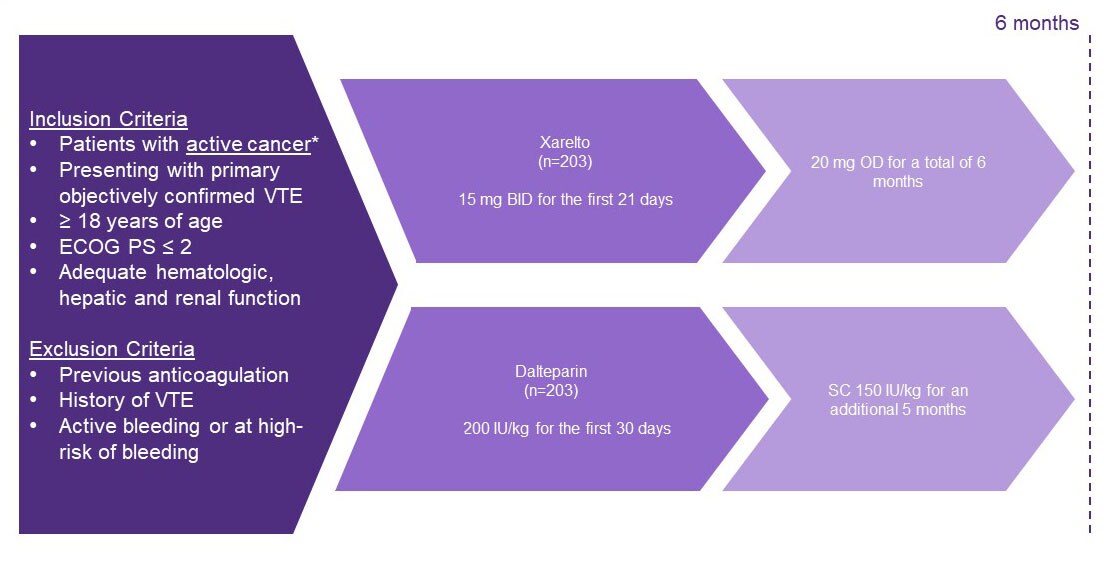
Study design1
- A multicentre, randomised, open-label, pilot trial in the United Kingdom to investigate patients with active cancer who had symptomatic PE, incidental PE or DVT. Patients were treated with either dalteparin or Xarelto
Endpoints1
Primary efficacy outcome
- VTE recurrence
Primary safety outcomes
- Major bleeding
- Clinically non-relevant bleeding
Key findings1
- Over the course of 6 months fewer patients treated with Xarelto experienced a recurrent VTE compared to dalteparin (4% vs 11.0% respectively).
- Only 54% of patients completed the 6-month duration of study with a median duration of treatment of 5.8 and 5.9 months for dalteparin and Xarelto respectively
- 4% of patients receiving dalteparin had major bleeds compared with 6% of patients treated with Xarelto.
- Incidence of fatal bleeding was the same in both groups (0.5%)
- 7 patients receiving dalteparin experienced CRNMB compared with 25 patients receiving Xarelto (3-fold relative increase).
- Overall survival at 6 months was 75% for Xarelto (95% CI 69%–81%) and 70% for dalteparin (95% CI 63%–76%).
BID, twice daily; ECOG PS, Eastern Cooperative Oncology Group performance status; LMWH, low-molecular weight heparin; OD, once daily; SC, subcutaneous; VTE, venous thromboembolism;
*Active cancer was defined as a diagnosis of cancer (other than basal-cell or squamous-cell skin carcinoma) within the previous 6 months, recurrent or metastatic cancer, or cancer not in complete remission.
PP-XAR-ALL-1826-1
References
- Young AM, et al. J Clin Oncol. 2018;36:2017–2023. Young AM, et al. J Clin Oncol. 2018;36:2017–2023. Return to content







